Drug Impurities Reference Standards
We offer a large selection of reference standards of drug-related process impurities, drug degradation products, including compendial impurities listed by USP, EP and other compendia. We strive to offer complete sets of drug impurities in line with analytical target profiles of drug substances. Many of our products are not available from any other manufacturer.
Showing 1–10 of 1879 results
-
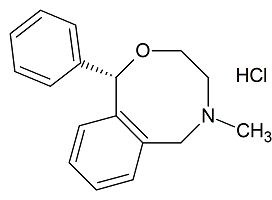
(+)-Nefopam Hydrochloride
- Product Number N-11203-01
- Parent Drug Nefopam
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
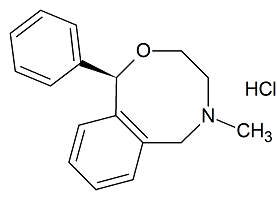
(-)-Nefopam Hydrochloride
- Product Number N-11203-02
- Parent Drug Nefopam
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
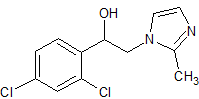
(2,4-Dichlorophenyl)-2-(2-methylimidazol-1-yl)ethanol
- Product Number I-10114-02
- Parent Drug Isoconazole
- CAS Number 57432-78-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
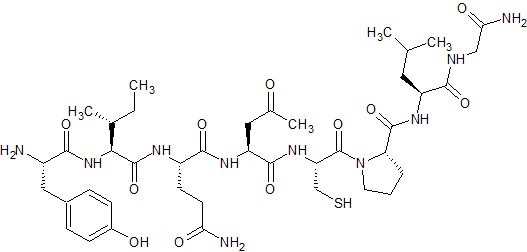
(2-9)-Oxytocin
- Product Number CT-01110-1108
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
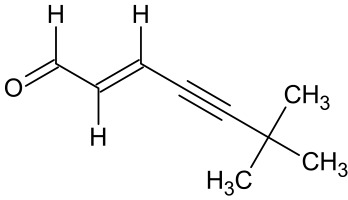
(2E)-6,6-dimethylhept-2-ene-4-ynal
- Product Number T-40111-01
- Parent Drug Terbinafine
- CAS Number 138139-82-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
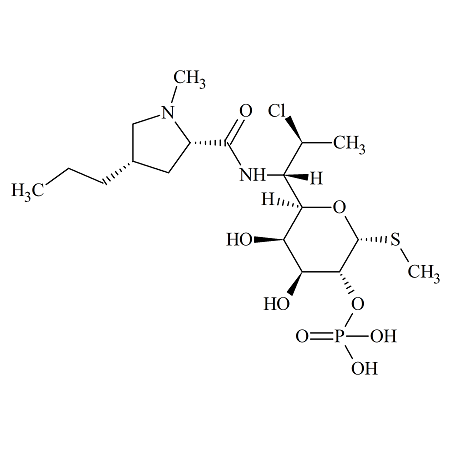
(2S-cis)-Clindamycin 2-Phosphate
- Product Number CLI-16-005
- Parent Drug Clindamycin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
(3-9)-Oxytocin
- Product Number CT-01110-1107
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
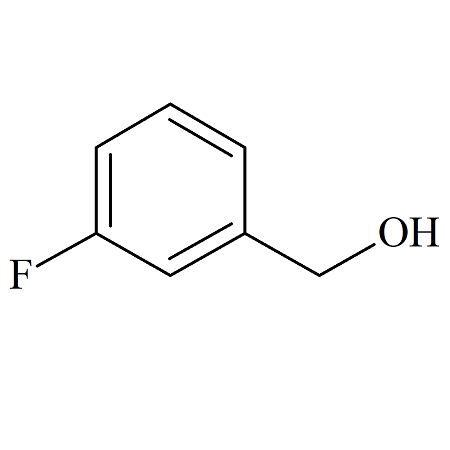
(3-Fluorophenyl)methanol Impurity Certified Reference Standard
- Product Number B-70915-0002
- Parent Drug Tavaborole
- CAS Number 456-47-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
(4-9)-Oxytocin
- Product Number CT-01110-1106
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
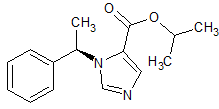
(R)-Etomidate EP Impurity C
- Product Number CT-01110-1055
- Parent Drug Etomidate
- CAS Number 771422-77-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options