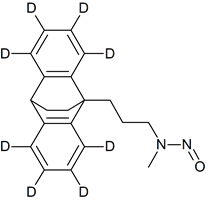Drug Impurities Reference Standards
Showing 1011–1020 of 1927 results
-
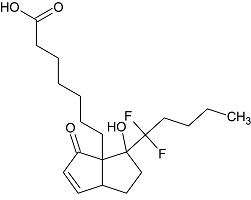
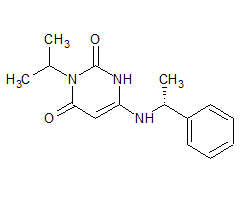
Lubiprostone Pericyclic Impurity
- Product Number L-11110-01
- Parent Drug Lubiprostone
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Lurasidone Impurity 23
- Product Number CT-01110-78
- Parent Drug Lurasidone
- CAS Number 186204-37-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
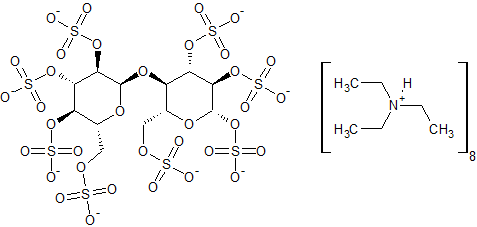
Maltose Octasulfate Potassium Salt
- Product Number S-90429-03
- Parent Drug Sucralfate
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Maprotyline-D8 N-nitroso
- Product Number M-40809-01
- Parent Drug Maprotyline
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
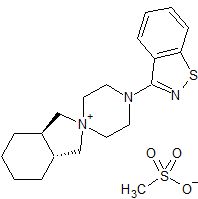
Mavacamten R-Enantiomer
- Product Number M-01106-2
- Parent Drug Mavacamten
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
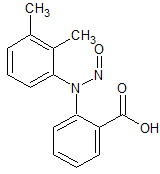
Mefenamic Acid N-Nitroso
- Product Number M-10312-01
- Parent Drug Mefenamic Acid
- CAS Number 2114-63-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
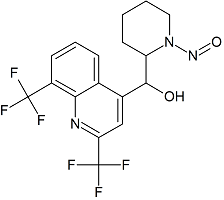
Mefloquine N-Nitroso
- Product Number M-21209-01
- Parent Drug Mefloquine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
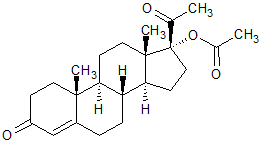
Megestrol Acetate EP Impurity K
- Product Number CT-01110-770
- Parent Drug Megestrol Acetate
- CAS Number 302-23-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
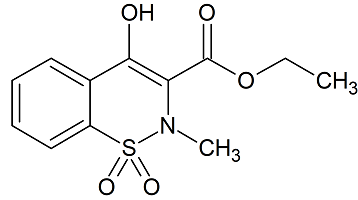
Meloxicam Related Compound A
- Product Number M-10205-01
- Parent Drug Meloxicam
- CAS Number 24683-26-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
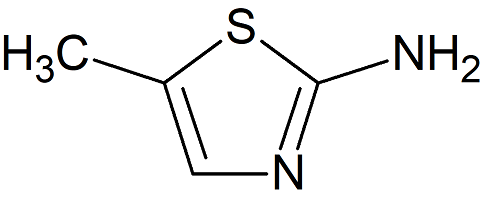
Meloxicam Related Compound B
- Product Number M-10205-02
- Parent Drug Meloxicam
- CAS Number 7305-71-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options