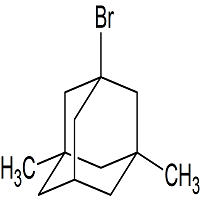
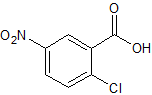
Drug Impurities Reference Standards
Showing 1021–1030 of 1927 results
-
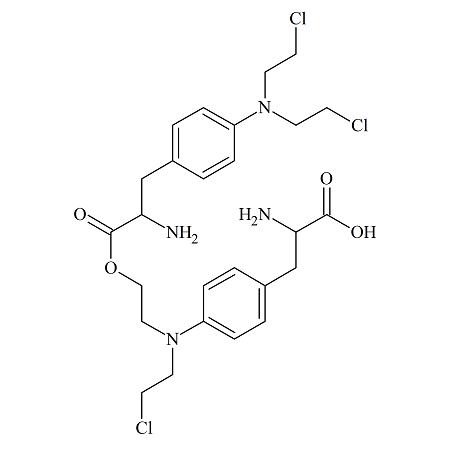
Melphalan dimer (Rac)
- Product Number MLP-16-001
- Parent Drug Melphalan
- CAS Number 148-82-3 (parent)
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 1 week(s)See more size options -
Memantine USP Related Compound D
- Product Number M-11018-01
- Parent Drug Memantine
- CAS Number 941-37-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
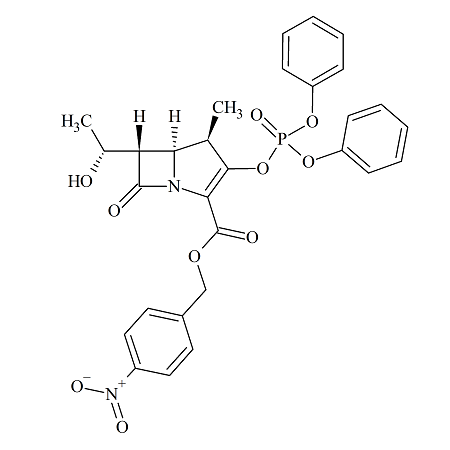
Meropenem Intermediate: 1ß-Methyl Carbapenem Derivative
- Product Number MRP-16-001
- Parent Drug Meropenem
- CAS Number 90776-59-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
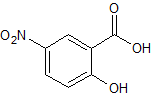
Mesalazine EP Impurity G
- Parent Drug Mesalamine
- CAS Number 490-79-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
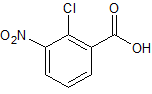
Mesalazine EP Impurity L
- Product Number ACA-161220-0008
- Parent Drug Mesalamine
- CAS Number 118-91-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mesalazine EP Impurity M
- Product Number CT-01110-631
- Parent Drug Mesalamine
- CAS Number 2516-96-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mesalazine EP Impurity N
- Product Number CT-01110-632
- Parent Drug Mesalamine
- CAS Number 96-97-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mesalazine EP Impurity Q
- Product Number CT-01110-633
- Parent Drug Mesalamine
- CAS Number 3970-35-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
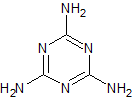
Metformin EP Impurity C
- Product Number CT-01110-547
- Parent Drug Metformin
- CAS Number 1985-46-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
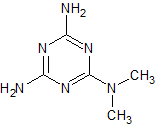
Metformin EP Impurity D
- Product Number CT-01110-548
- Parent Drug Metformin
- CAS Number 108-78-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options