Drug Impurities Reference Standards
Showing 1171–1180 of 1927 results
-
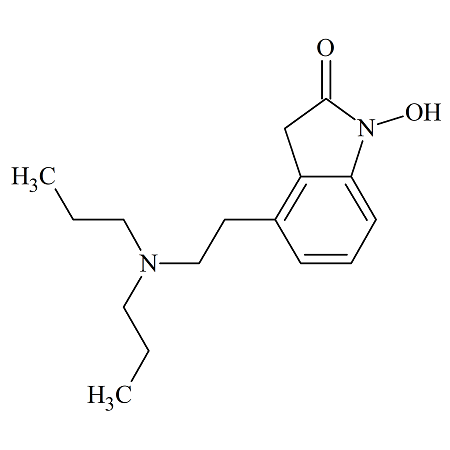
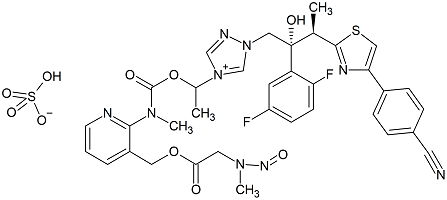
N-Nitroso Isavuconazonium Sulfate
- Product Number I-30501-01
- Parent Drug Isavuconazonium Sulfate
- CAS Number NA
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
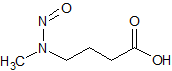
N-nitroso-N-methyl-4-aminobutanoic acid (NMBA)
- Product Number N-10521-08
- Parent Drug Nitroso Compounds
- CAS Number 61445-55-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
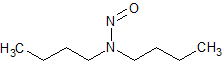
N-nitrosodibutylamine (NDBA)
- Product Number N-10521-02
- Parent Drug Nitroso Compounds
- CAS Number 924-16-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
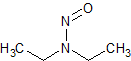
N-nitrosodiethylamine (NDEA)
- Product Number N-10521-03
- Parent Drug Nitroso Compounds
- CAS Number 55-18-5
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
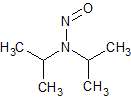
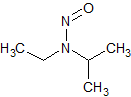
N-nitrosodiisopropylamine (NDIPA)
- Product Number N-10521-04
- Parent Drug Nitroso Compounds
- CAS Number 601-77-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
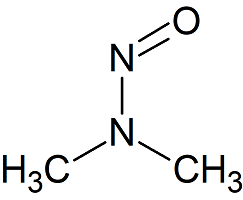
N-nitrosodimethylamine (NDMA)
- Product Number N-10521-05
- Parent Drug Nitroso Compounds
- CAS Number 62-75-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
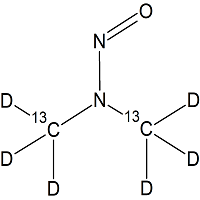
N-Nitrosodimethylamine – 13C2D6
- Product Number FCD-11013-01
- Parent Drug Nitroso Compounds
- CAS Number 62-75-9 (unlabeled)
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
N-nitrosoisopropylethyl amine (NIPEA)
- Product Number N-10521-06
- Parent Drug Nitroso Compounds
- CAS Number 16339-04-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
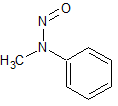
N-nitrosomethylphenylamine (NMPA)
- Product Number N-10521-07
- Parent Drug Nitroso Compounds
- CAS Number 614-00-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options