Drug Impurities Reference Standards
Showing 1181–1190 of 1927 results
-
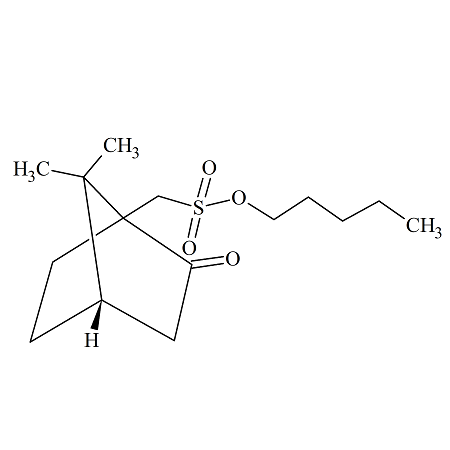
n-Pentyl (+/-)-10-Camphorsulfonate
- Product Number CAM-14-001
- Parent Drug Camphorsulfonates
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
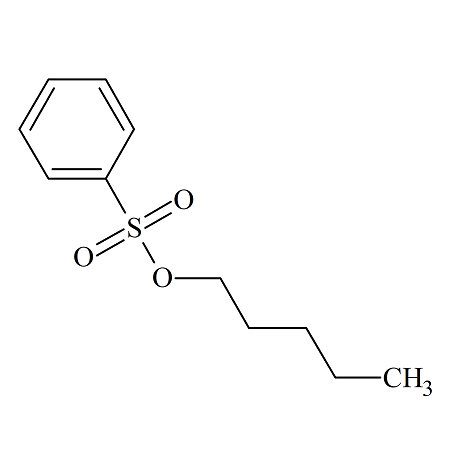
n-Pentyl Benzenesulfonate
- Product Number BES-12-002
- Parent Drug Benzenesulfonates
- CAS Number 80-45-5
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
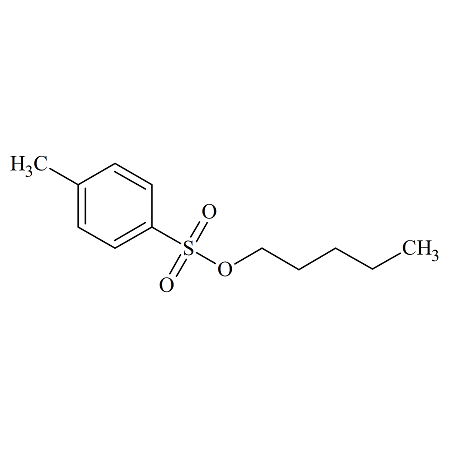
n-Pentyl Toluenesulfonate
- Product Number TOS-12-002
- Parent Drug Toluenesulfonates
- CAS Number 4450-76-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
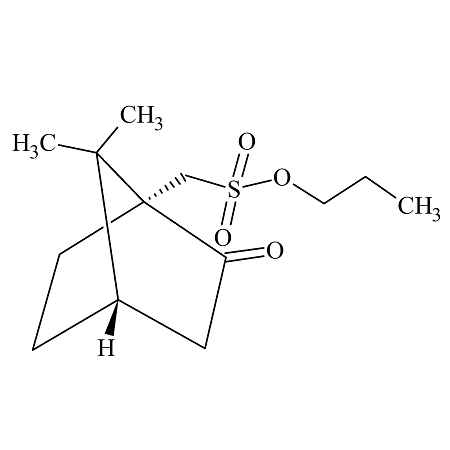
n-Propyl (+/-)-10-Camphorsulfonate
- Product Number CAM-09-003
- Parent Drug Camphorsulfonates
- CAS Number 1242184-38-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
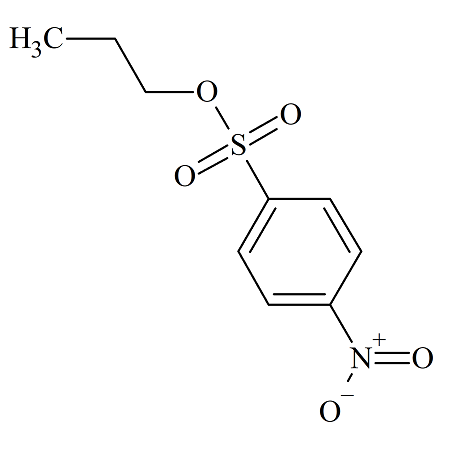
n-Propyl 4-Nitrobenzenesulfonate
- Product Number NBS-10-003
- Parent Drug p-Nitrobenzenesulfonates
- CAS Number 33420-15-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
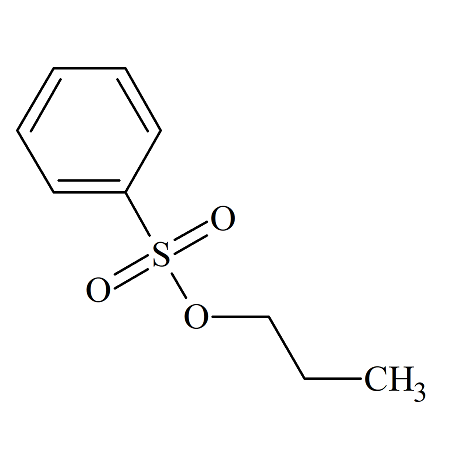
n-Propyl Benzenesulfonate
- Product Number BES-09-001
- Parent Drug Benzenesulfonates
- CAS Number 80-42-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
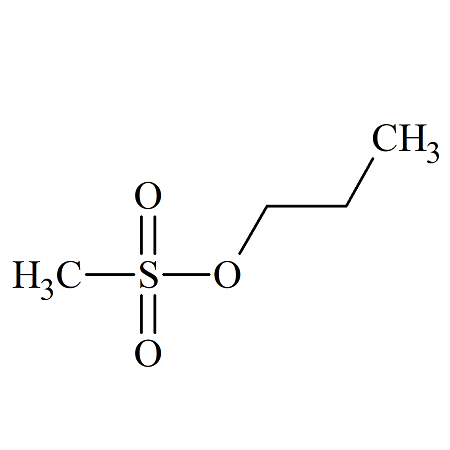
n-Propyl Methanesulfonate
- Product Number MET-09-003
- Parent Drug Methanesulfonates
- CAS Number 1912-31-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
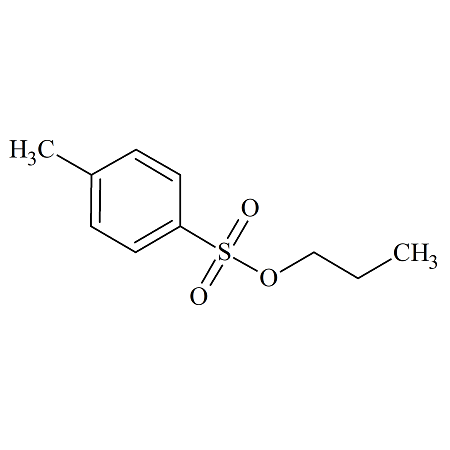
n-Propyl p-Toluenesulfonate
- Product Number TOS-09-003
- Parent Drug Toluenesulfonates
- CAS Number 599-91-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
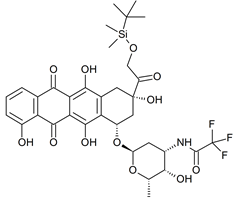
N-trifluoroacetyl-14-O-tert-butyldimethylsilyl-4-desmethyl-doxorubicin
- Product Number D-50710-01
- Parent Drug Doxorubicin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
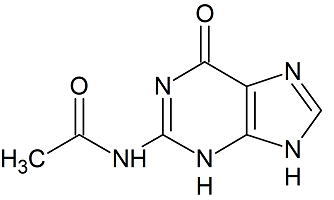
N2-Acetylguanine
- Product Number A-20112-02
- Parent Drug Acyclovir
- CAS Number 19962-37-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options