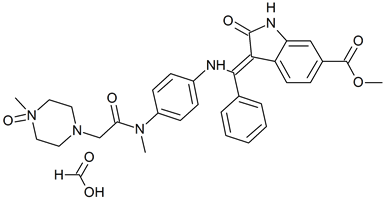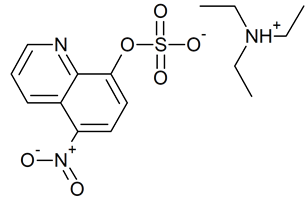Drug Impurities Reference Standards
Showing 1241–1250 of 1927 results
-
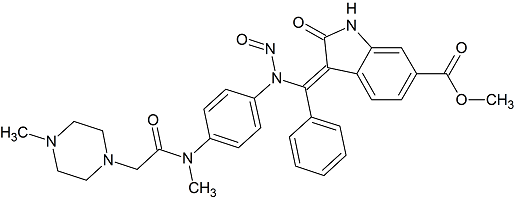
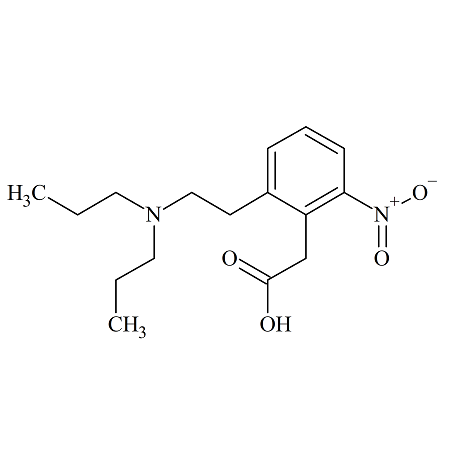
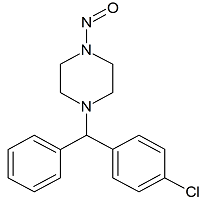
Nintedanib N-nitroso
- Product Number N-30908-01
- Parent Drug Nintedanib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
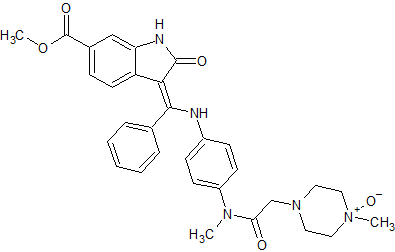
Nintedanib N-Oxide
- Product Number N-01029-01
- Parent Drug Nintedanib
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Nintedanib N-Oxide Formate Salt
- Product Number N-40605-01
- Parent Drug Nintedanib
- CAS Number 2734666-06-7 (free base)
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
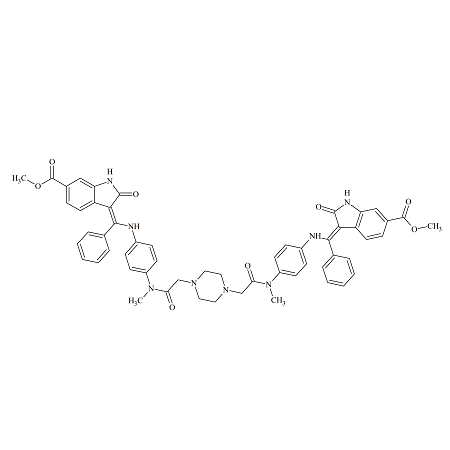
Nintedanib Piperazine Dimer Impurity
- Product Number B-71120-0007
- Parent Drug Nintedanib
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Nitro Ropinirole
- Product Number ROP-10-002
- Parent Drug Ropinirole
- CAS Number 91374-25-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Nitroxoline sulfate triethylamine salt
- Product Number N-40523-01
- Parent Drug Nitroxoline
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
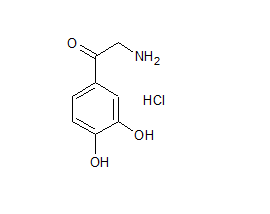
Noradrenalone HCl
- Product Number N-90111-01
- Parent Drug Norepinephrine
- CAS Number 5090-29-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
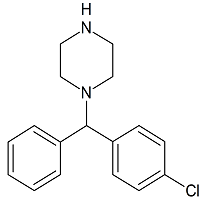
Norchlorcyclizine
- Product Number C-10921-01
- Parent Drug Chlorcyclizine
- CAS Number 303-26-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Norchlorcyclizine N-Nitroso
- Product Number C-10921-02
- Parent Drug Chlorcyclizine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
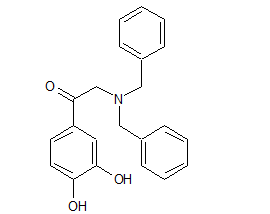
Norepinephrine Dibenzylamino Impurity
- Product Number N-90111-03
- Parent Drug Norepinephrine
- CAS Number 13062-58-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options