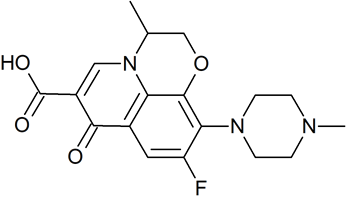
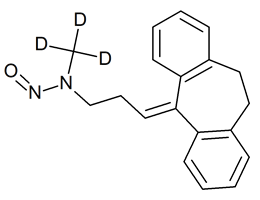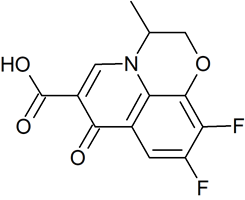Drug Impurities Reference Standards
Showing 1251–1260 of 1927 results
-
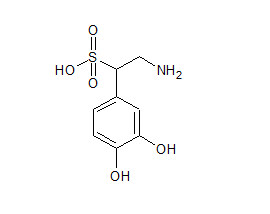
Norepinephrine Sulfonic Acid
- Product Number N-90111-02
- Parent Drug Norepinephrine
- CAS Number 24159-36-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
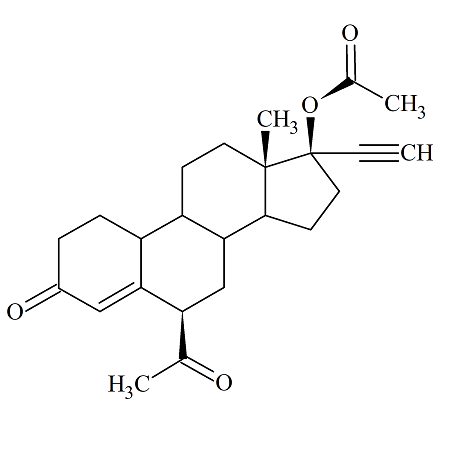
Norethindrone Acetate Impurity D EP; 6ß-Acetyl Norethindrone Acetate
- Product Number ACA-160926-0024
- Parent Drug Norethindrone
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
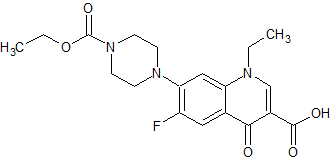
Norfloxacin EP Impurity H
- Product Number CT-01110-820
- Parent Drug Norfloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
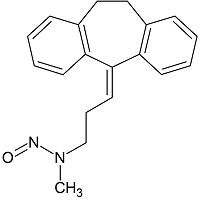
Nortriptyline N-Nitroso
- Product Number N-20301-01
- Parent Drug Nortriptyline
- CAS Number 55855-42-0
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: week(s)See more size options -
Nortriptyline-D3 N-nitroso
- Product Number N-40809-01
- Parent Drug Nortriptyline
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
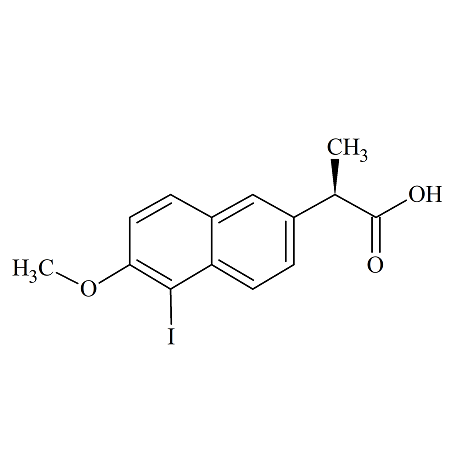
O-Bromo Naproxen
- Product Number NAP-16-004
- Parent Drug Naproxen
- CAS Number 5111-65-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
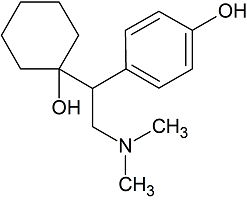
O-Desmethylvenlafaxine
- Product Number V-21116-01
- Parent Drug Venlafaxine
- CAS Number 93413-62-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
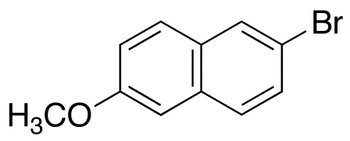
O-Iodo Naproxen
- Product Number NAP-16-005
- Parent Drug Naproxen
- CAS Number 116883-61-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ofloxacine
- Product Number O-50416-01
- Parent Drug Ofloxacine
- CAS Number 82419-36-1
- Category Drug Impurities Reference Standards
-
Ofloxacine Impurity A
- Product Number O-50416-02
- Parent Drug Ofloxacine
- CAS Number 82419-35-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options