Drug Impurities Reference Standards
Showing 1261–1270 of 1927 results
-
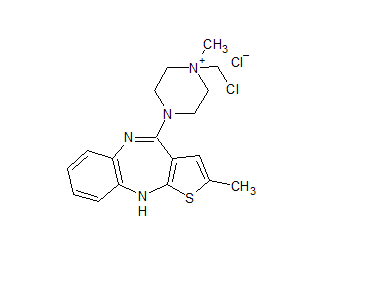
Olanzapine Chloromethyl Chloride Impurity
- Product Number OLA-20-001
- Parent Drug Olanzapine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
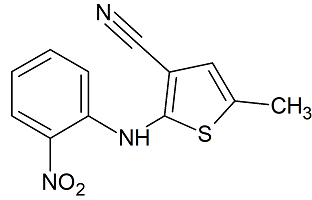
Olanzapine Impurity 11
- Product Number CT-01110-507
- Parent Drug Olanzapine
- CAS Number 1017241-36-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Olanzapine Lactam
- Product Number OLA-14-002
- Parent Drug Olanzapine
- CAS Number 1017241-34-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Olanzapine Related Compound A
- Product Number O-10208-01
- Parent Drug Olanzapine
- CAS Number 138564-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Olanzapine Related Compound C
- Product Number O-10208-02
- Parent Drug Olanzapine
- CAS Number 174794-02-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
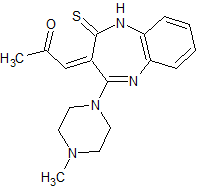
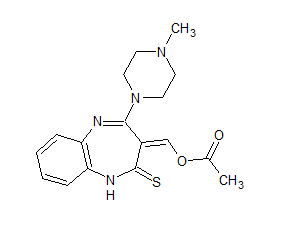
Olanzapine Thioacetoxymethylidene
- Product Number OLA-19-001
- Parent Drug Olanzapine
- CAS Number 1320360-87-9
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
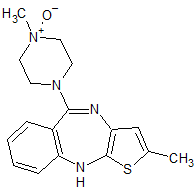
Olanzapine Thiolactam
- Product Number OLA-14-003
- Parent Drug Olanzapine
- CAS Number 1017241-36-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
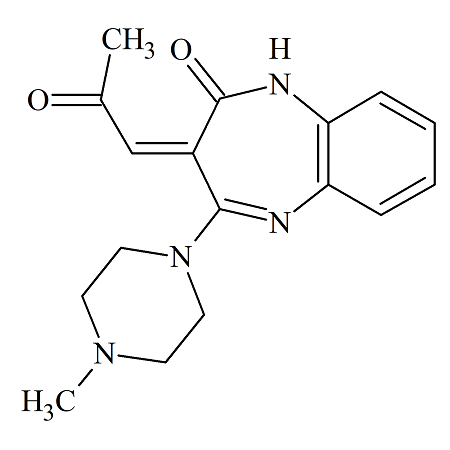
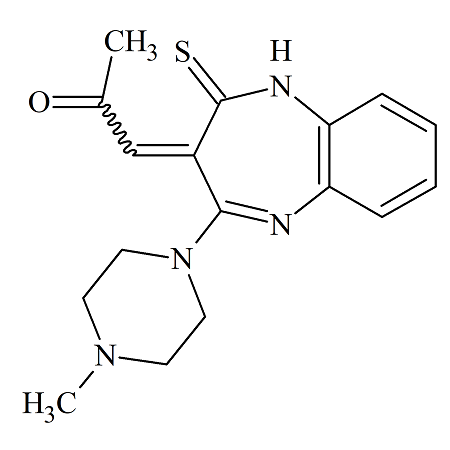
Olanzapine Thiooxymethylidene
- Product Number OLA-19-002
- Parent Drug Olanzapine
- CAS Number N/A
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
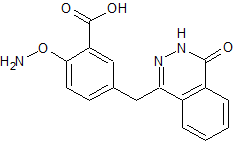
Olaparib Aminooxy Despiperazine Impurity
- Product Number O-00521-03
- Parent Drug Olaparib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
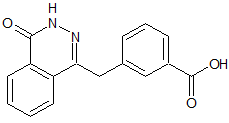
Olaparib Desfluoro Despiperazine Impurity
- Product Number O-90410-3
- Parent Drug Olaparib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options