Drug Impurities Reference Standards
Showing 1291–1300 of 1927 results
-
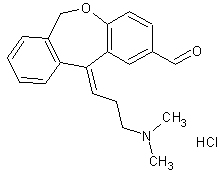
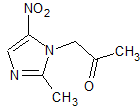
Olopatadine Carbaldehyde (as Hydrochloride)
- Product Number OLO-11-004
- Parent Drug Olopatadine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
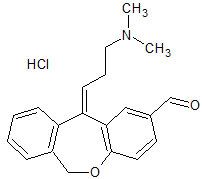
Olopatadine Impurity 4
- Product Number CT-01110-958
- Parent Drug Olopatadine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
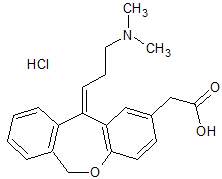
Olopatadine Impurity 7
- Product Number CT-01110-959
- Parent Drug Olopatadine
- CAS Number 949141-22-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ondansetron EP Impurity F
- Product Number CT-01110-728
- Parent Drug Ondansetron
- CAS Number 693-98-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
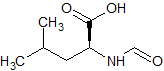
Orlistat Impurity 5
- Product Number CT-01110-845
- Parent Drug Orlistat I
- CAS Number 791-28-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
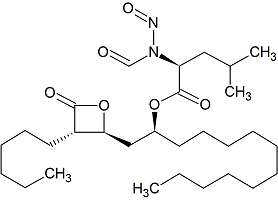
Orlistat Impurity 7
- Product Number CT-01110-846
- Parent Drug Orlistat I
- CAS Number 6113-61-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
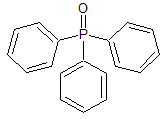
Orlistat N-Nitroso
- Product Number O-30315-01
- Parent Drug Orlistat
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Ornidazole Impurity 11
- Product Number CT-01110-637
- Parent Drug Ornidazole
- CAS Number 106-89-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ornidazole Impurity 2
- Product Number CT-01110-638
- Parent Drug Ornidazole
- CAS Number 31876-69-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
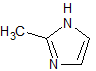
Ornidazole Impurity 3
- Product Number CT-01110-639
- Parent Drug Ornidazole
- CAS Number 16773-52-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options