Drug Impurities Reference Standards
Showing 1351–1360 of 1927 results
-
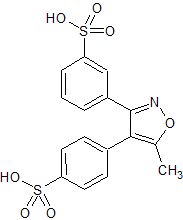
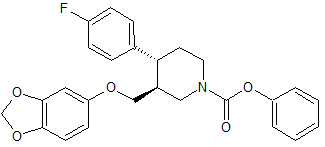
Parecoxib Sodium Impurity 26
- Product Number CT-01110-886
- Parent Drug Parecoxib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
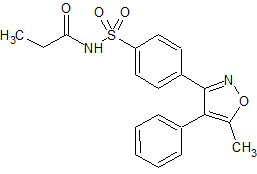
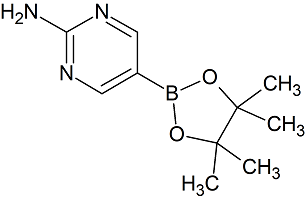
Parecoxib sodium Impurity 40
- Product Number CT-01110-887
- Parent Drug Parecoxib
- CAS Number 2235371-89-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
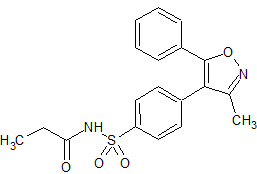
Parecoxib Sodium Impurity 46
- Product Number CT-01110-888
- Parent Drug Parecoxib
- CAS Number 477594-28-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
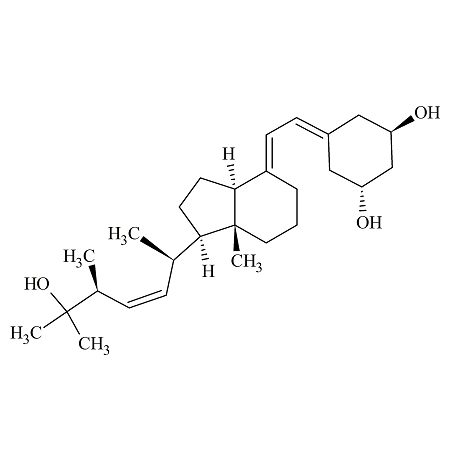
Paricalcitol 22-Z
- Product Number PAR-16-001
- Parent Drug Paricalcitol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
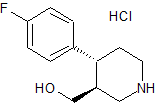
Paroxetine EP Impurity G
- Product Number CT-01110-92
- Parent Drug Paroxetine
- CAS Number 1012886-75-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
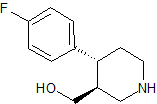
Paroxetine EP Impurity I
- Product Number CT-01110-90
- Parent Drug Paroxetine
- CAS Number 125224-43-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
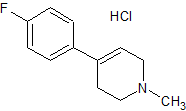
Paroxetine EP Impurity I HCl
- Product Number CT-01110-91
- Parent Drug Paroxetine
- CAS Number 220548-73-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
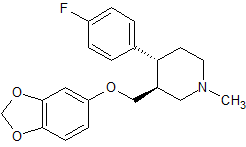
Paroxetine Impurity 29
- Product Number CT-01110-88
- Parent Drug Paroxetine
- CAS Number 110429-36-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Paroxetine Impurity 30
- Product Number CT-01110-89
- Parent Drug Paroxetine
- CAS Number 253768-88-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Paxalisib Impurity G00050050
- Product Number P-10122-01
- Parent Drug Paxalisib (GDC-0084)
- CAS Number 402960-38-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options