Drug Impurities Reference Standards
Showing 1431–1440 of 1927 results
-
Prasugrel Impurity 3
- Product Number CT-01110-254
- Parent Drug Prasugrel
- CAS Number 1391194-39-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
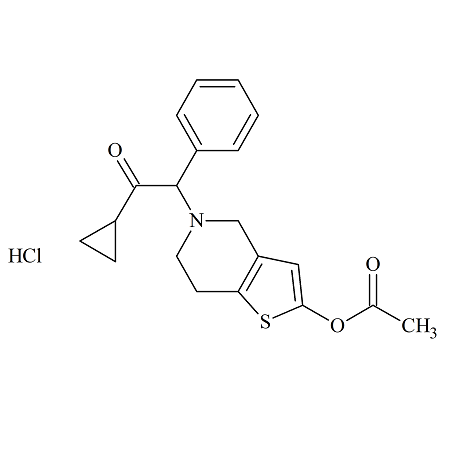
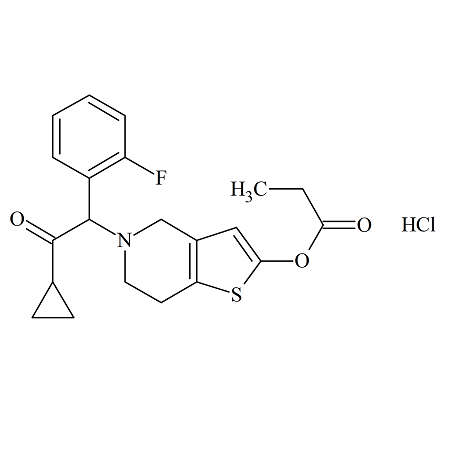
Prasugrel Propionate Analog Hydochloride
- Product Number ACB-170111-0007
- Parent Drug Prasugrel
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
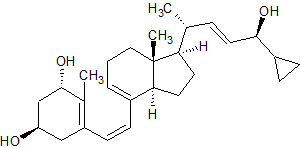
Pre-Calcipotriol
- Product Number C-00116-02
- Parent Drug Calcipotriol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
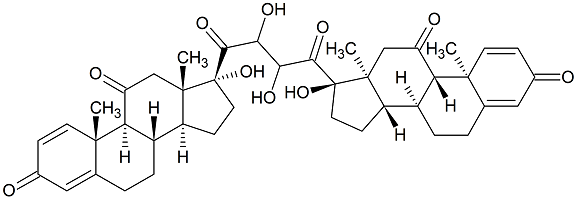
Prednisone Impurity 6
- Product Number P-20526-01
- Parent Drug Prednisone
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Pregabalin EP Impurity A(Pregabalin USP impurity C)
- Product Number CT-01110-622
- Parent Drug Pregabalin
- CAS Number 181289-23-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pregabalin Impurity 12
- Product Number CT-01110-620
- Parent Drug Pregabalin
- CAS Number 216576-74-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
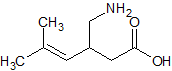
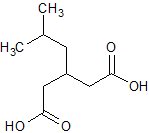
Pregabalin Impurity 2(3-IsobutylglutaricAcid)
- Product Number CT-01110-621
- Parent Drug Pregabalin
- CAS Number 75143-89-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
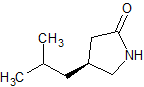
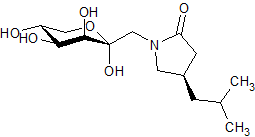
Pregabalin Impurity PD 0224377
- Product Number CT-01110-624
- Parent Drug Pregabalin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options