Drug Impurities Reference Standards
Showing 1631–1640 of 1927 results
-
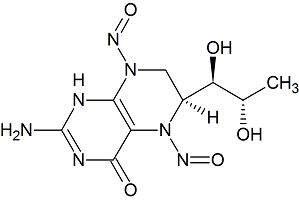
Sapropterin N-Nitroso Impurity 3
- Product Number S-30201-03
- Parent Drug Sapropterin
- CAS Number N/A
- Category Drug Impurities Reference Standards
DiscontinuedSee more size options -
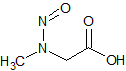
Sarcosine N-Nitroso
- Product Number S-10521-01
- Parent Drug Sarcosine
- CAS Number 13256-22-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
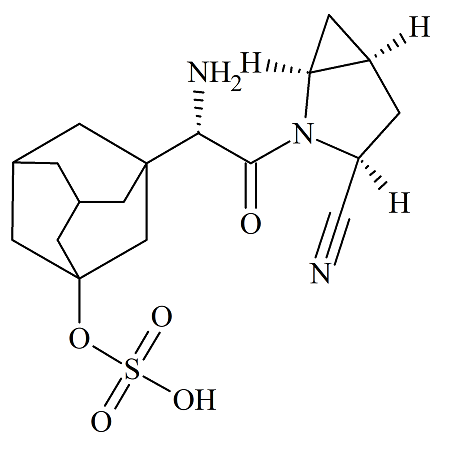
Saxagliptin O-Sulfate
- Product Number SXG-15-004
- Parent Drug Saxagliptin
- CAS Number 1429782-94-4
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
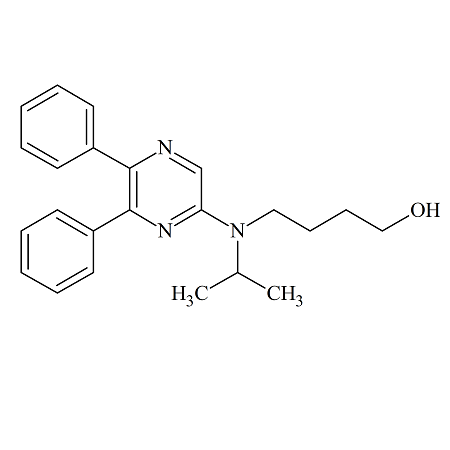
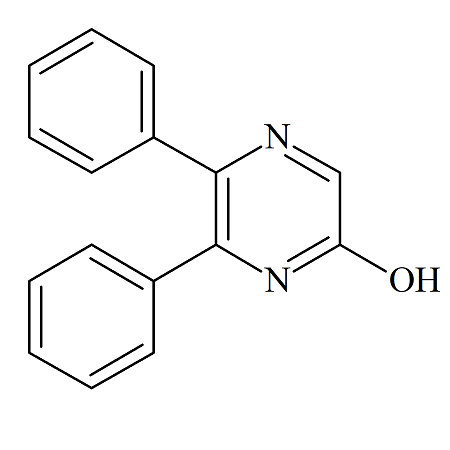
Selexipag Impurity A
- Product Number ACB-161114-0004
- Parent Drug Selexipag
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
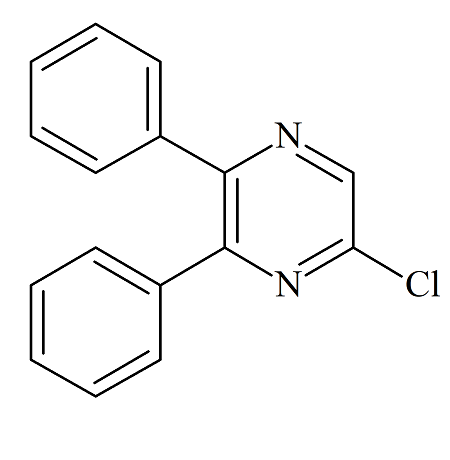
Selexipag Impurity B
- Product Number ACB-161114-0005
- Parent Drug Selexipag
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
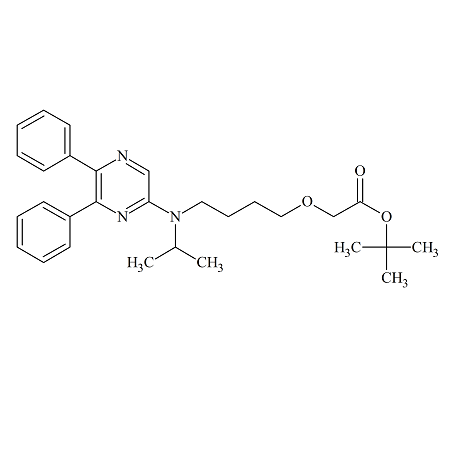
Selexipag Impurity C
- Product Number ACB-161114-0006
- Parent Drug Selexipag
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
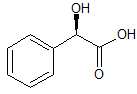
Selexipag Impurity D
- Product Number ACB-161114-0007
- Parent Drug Selexipag
- CAS Number 475084-96-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Sertraline EP Impurity E
- Product Number CT-01110-650
- Parent Drug Sertraline
- CAS Number 611-71-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
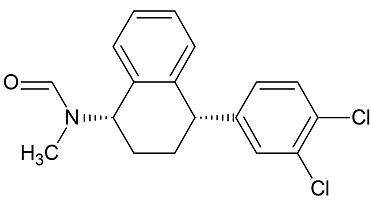
Sertraline N-formyl
- Product Number S-31107-01
- Parent Drug Sertraline
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
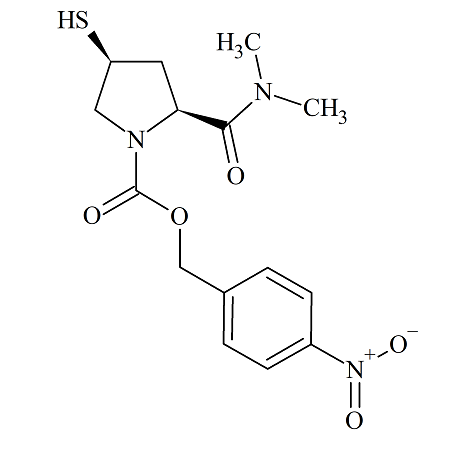
Side chain for Meropenem(2S-CIS)
- Product Number MRP-16-003
- Parent Drug Meropenem
- CAS Number 96034-64-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options