Drug Impurities Reference Standards
Showing 1651–1660 of 1927 results
-
Sitagliptin Impurity D
- Product Number CT-01110-01
- Parent Drug Sitagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
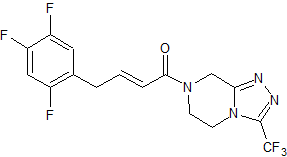
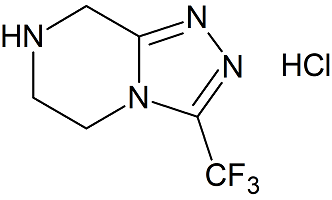
Sitagliptin Intermediates Impurity
- Product Number CT-01110-439
- Parent Drug Sitagliptin
- CAS Number 762240-92-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
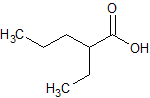
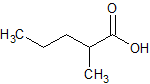
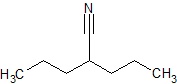
Sodium Valproate EP Impurity B
- Product Number CT-01110-486
- Parent Drug Valproic Acid
- CAS Number 20225-24-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
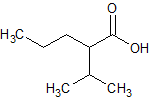
Sodium Valproate EP Impurity C
- Product Number CT-01110-484
- Parent Drug Valproic Acid
- CAS Number 62391-99-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Sodium Valproate EP Impurity D
- Product Number CT-01110-489
- Parent Drug Valproic Acid
- CAS Number 52061-75-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
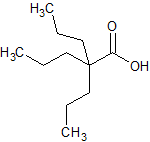
Sodium Valproate EP Impurity F
- Product Number CT-01110-479
- Parent Drug Valproic Acid
- CAS Number 2430-27-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
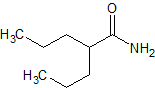
Sodium Valproate EP Impurity G
- Product Number CT-01110-480
- Parent Drug Valproic Acid
- CAS Number 52061-73-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
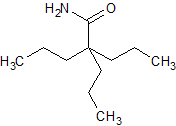
Sodium Valproate EP Impurity L
- Product Number CT-01110-485
- Parent Drug Valproic Acid
- CAS Number 97-61-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Sodium Valproate Impurity 20
- Product Number CT-01110-481
- Parent Drug Valproic Acid
- CAS Number 13310-75-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
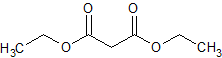
Sodium Valproate Impurity 22
- Product Number CT-01110-483
- Parent Drug Valproic Acid
- CAS Number 105-53-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options