Drug Impurities Reference Standards
Showing 1771–1780 of 1927 results
-
Tenofovir Disoproxil Impurity 35
- Product Number CT-01110-219
- Parent Drug Tenofovir
- CAS Number 14047-27-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
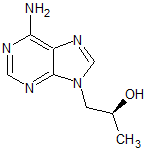
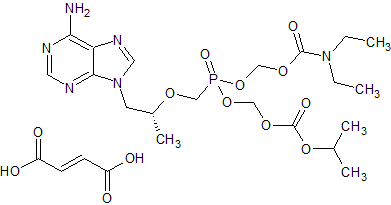
Tenofovir Disoproxil Impurity 4
- Product Number CT-01110-220
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
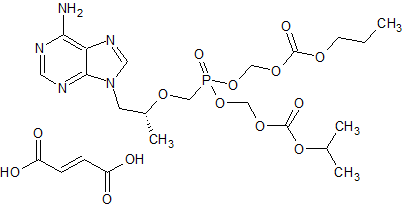
Tenofovir disoproxil Impurity 6
- Product Number CT-01110-222
- Parent Drug Tenofovir
- CAS Number 1093279-77-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
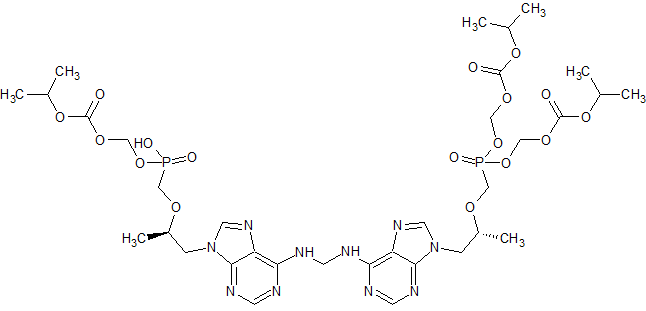
Tenofovir disoproxil Impurity 7
- Product Number CT-01110-223
- Parent Drug Tenofovir
- CAS Number 1422284-16-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
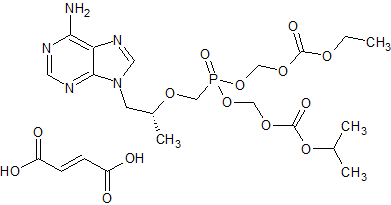
Tenofovir disoproxil Impurity 8
- Product Number CT-01110-224
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Terbinafine EP Impurity A
- Product Number T-30117-01
- Parent Drug Terbinafine
- CAS Number 65473-13-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
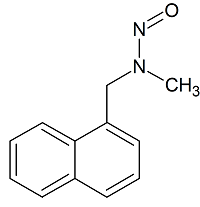
Terbinafine EP Impurity A N-Nitroso
- Product Number T-30117-02
- Parent Drug Terbinafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
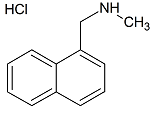
Terbinafine HCl Impurity
- Product Number T-00702-01
- Parent Drug Terbinafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
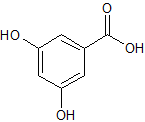
Terbutaline EP Impurity A
- Product Number CT-01110-509
- Parent Drug Terbutaline
- CAS Number 36438
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
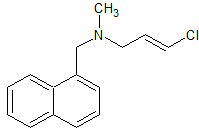
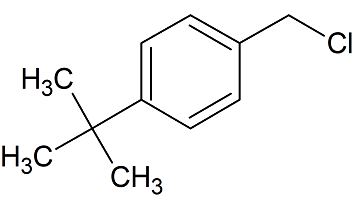
tert-Butylbenzyl Chloride
- Product Number B-11029-08
- Parent Drug Butenafine
- CAS Number 19692-45-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options