Drug Impurities Reference Standards
Showing 1791–1800 of 1927 results
-
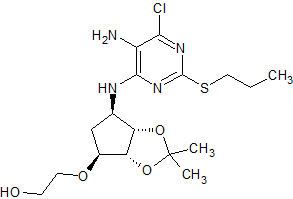
Ticagrelor Impurity 47
- Product Number CT-01110-318
- Parent Drug Ticagrelor
- CAS Number 376608-74-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
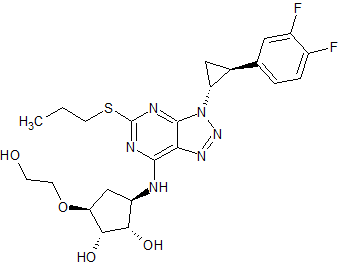
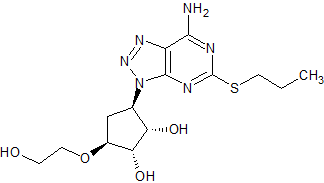
Ticagrelor Impurity 5
- Product Number CT-01110-319
- Parent Drug Ticagrelor
- CAS Number 1788033-05-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
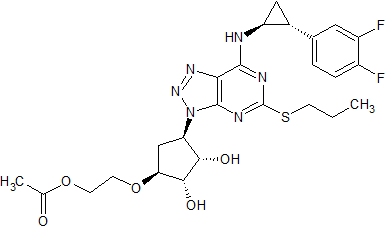
Ticagrelor Impurity 6
- Product Number CT-01110-320
- Parent Drug Ticagrelor
- CAS Number 1616703-93-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
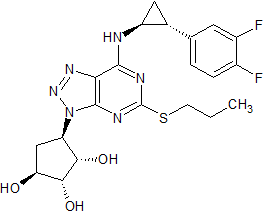
Ticagrelor Impurity 7
- Product Number CT-01110-321
- Parent Drug Ticagrelor
- CAS Number 220347-05-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Ticagrelor Impurity 8
- Product Number CT-01110-322
- Parent Drug Ticagrelor
- CAS Number 1251765-07-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
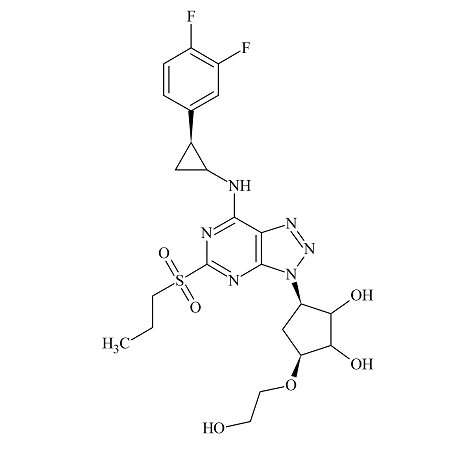
Ticagrelor Sulfone
- Product Number TIC-16-001
- Parent Drug Ticagrelor
- CAS Number 274693-39-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
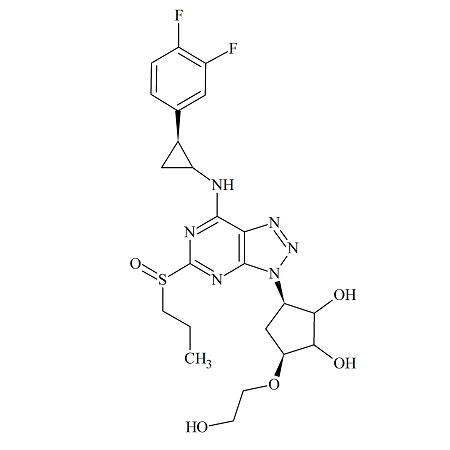
Ticagrelor Sulfoxide Impurity (Mixture of diastereomers)
- Product Number TIC-16-002
- Parent Drug Ticagrelor
- CAS Number 1644461-85-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Timolol EP Impurity F
- Product Number CT-01110-844
- Parent Drug Timolol
- CAS Number 30165-96-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
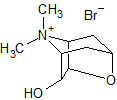
Tiotropium Bromide EP Impurity H (Mixture of Enantiomers)
- Product Number CT-01110-978
- Parent Drug Tiotropium
- CAS Number 1044148-31-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
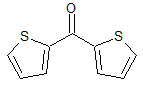
Tiotropium EP Impurity F
- Product Number CT-01110-976
- Parent Drug Tiotropium
- CAS Number 704-38-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options