Drug Impurities Reference Standards
Showing 1801–1810 of 1927 results
-
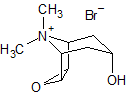
Tiotropium EP Impurity G
- Product Number CT-01110-977
- Parent Drug Tiotropium
- CAS Number 1508-46-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
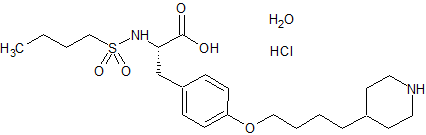
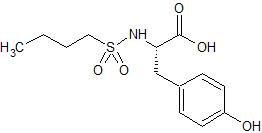
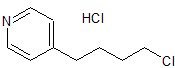
Tirofiban hydrochloride
- Product Number CT-01110-600
- Parent Drug Tirofiban
- CAS Number 150915-40-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
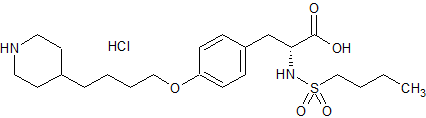
Tirofiban Impurity 2
- Product Number CT-01110-601
- Parent Drug Tirofiban
- CAS Number 158808-86-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
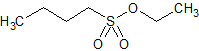
Tirofiban Impurity 22
- Product Number CT-01110-602
- Parent Drug Tirofiban
- CAS Number 2374-68-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 23
- Product Number CT-01110-603
- Parent Drug Tirofiban
- CAS Number 149490-60-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 26
- Product Number CT-01110-604
- Parent Drug Tirofiban
- CAS Number 102878-73-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 29
- Product Number CT-01110-605
- Parent Drug Tirofiban
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 31
- Product Number CT-01110-606
- Parent Drug Tirofiban
- CAS Number 57614-92-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tirofiban Impurity 40
- Product Number CT-01110-607
- Parent Drug Tirofiban
- CAS Number 149463-65-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
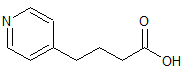
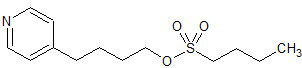
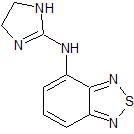
Tizanidine EP Impurity A
- Product Number CT-01110-810
- Parent Drug Tizanidine
- CAS Number 51322-69-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options