Drug Impurities Reference Standards
Showing 1841–1850 of 1879 results
-
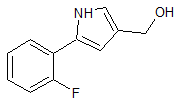
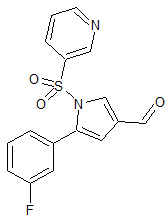
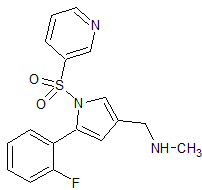
Vonoprazan Fumarate Impurity 33
- Product Number CT-01110-54
- Parent Drug Vonoprazan
- CAS Number 881674-58-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
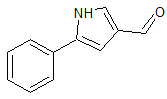
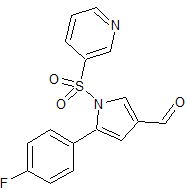
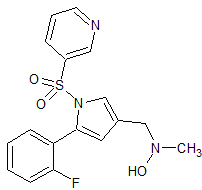
Vonoprazan Fumarate impurity 36
- Product Number CT-01110-55
- Parent Drug Vonoprazan
- CAS Number 56448-22-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
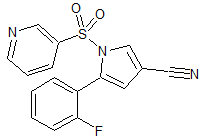
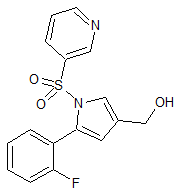
Vonoprazan Fumarate Impurity 4
- Product Number CT-01110-56
- Parent Drug Vonoprazan
- CAS Number 1807642-39-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
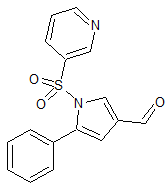
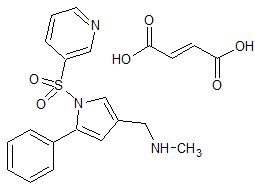
Vonoprazan Fumarate Impurity 40
- Product Number CT-01110-57
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 41
- Product Number CT-01110-58
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 42
- Product Number CT-01110-59
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 43
- Product Number CT-01110-60
- Parent Drug Vonoprazan
- CAS Number 2169271-28-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 45
- Product Number CT-01110-61
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate impurity 47
- Product Number CT-01110-62
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 48
- Product Number CT-01110-63
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options