Drug Impurities Reference Standards
Showing 1881–1890 of 1927 results
-
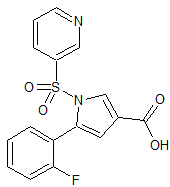
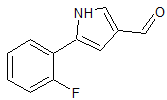
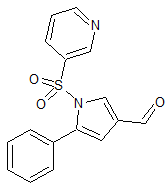
Vonoprazan Fumarate Impurity 18
- Product Number CT-01110-49
- Parent Drug Vonoprazan
- CAS Number 1883595-37-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
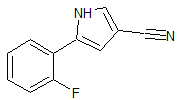
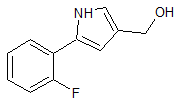
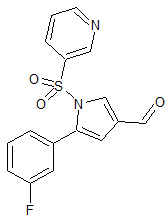
Vonoprazan Fumarate Impurity 2
- Product Number CT-01110-50
- Parent Drug Vonoprazan
- CAS Number 1240948-77-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
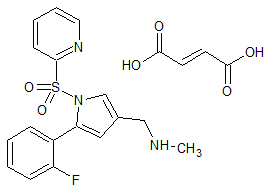
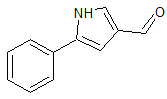
Vonoprazan Fumarate Impurity 23
- Product Number CT-01110-51
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
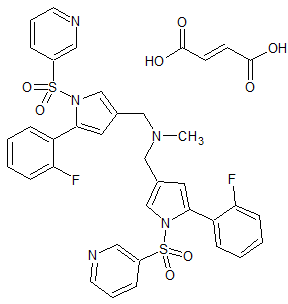
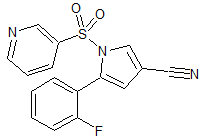
Vonoprazan Fumarate Impurity 28
- Product Number CT-01110-52
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 3
- Product Number CT-01110-53
- Parent Drug Vonoprazan
- CAS Number 881674-56-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 33
- Product Number CT-01110-54
- Parent Drug Vonoprazan
- CAS Number 881674-58-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate impurity 36
- Product Number CT-01110-55
- Parent Drug Vonoprazan
- CAS Number 56448-22-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 4
- Product Number CT-01110-56
- Parent Drug Vonoprazan
- CAS Number 1807642-39-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 40
- Product Number CT-01110-57
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 41
- Product Number CT-01110-58
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options