Drug Impurities Reference Standards
Showing 1891–1900 of 1927 results
-
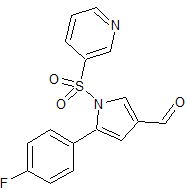
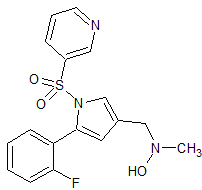
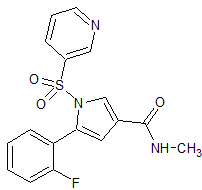
Vonoprazan Fumarate Impurity 42
- Product Number CT-01110-59
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
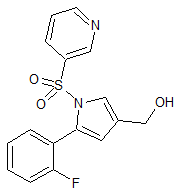
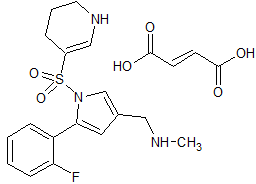
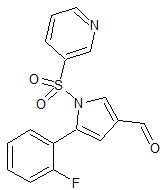
Vonoprazan Fumarate Impurity 43
- Product Number CT-01110-60
- Parent Drug Vonoprazan
- CAS Number 2169271-28-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
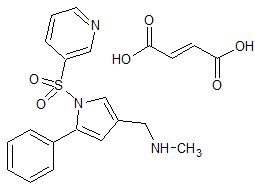
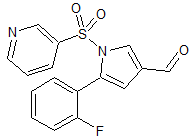
Vonoprazan Fumarate Impurity 45
- Product Number CT-01110-61
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
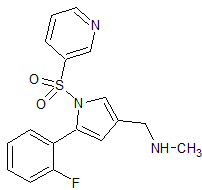
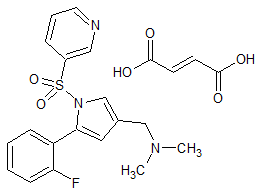
Vonoprazan Fumarate impurity 47
- Product Number CT-01110-62
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 48
- Product Number CT-01110-63
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 49
- Product Number CT-01110-64
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 5
- Product Number CT-01110-65
- Parent Drug Vonoprazan
- CAS Number 881677-11-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 52
- Product Number CT-01110-66
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 58
- Product Number CT-01110-67
- Parent Drug Vonoprazan
- CAS Number 2054536-04-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate Impurity 64
- Product Number CT-01110-68
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options