Drug Impurities Reference Standards
Showing 191–200 of 1927 results
-
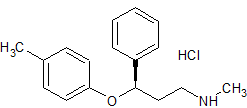
Atomoxetine EP Impurity C HCl
- Product Number CT-01110-866
- Parent Drug Atomoxetine
- CAS Number 1643684-06-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
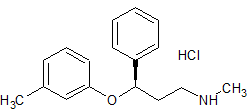
Atomoxetine EP Impurity D HCl
- Product Number CT-01110-867
- Parent Drug Atomoxetine
- CAS Number 873310-28-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
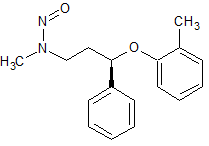
Atomoxetine N-Nitroso
- Product Number A-10521-04
- Parent Drug Atomoxetine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
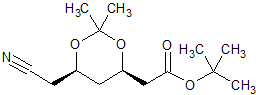
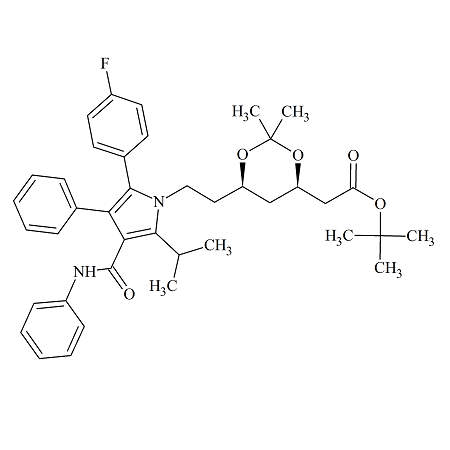
Atorvastatin Acetonide t-Butyl Ester Side Chain (4R,6R)-Isomer
- Product Number CT-01110-417
- Parent Drug Atorvastatin
- CAS Number 125971-94-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
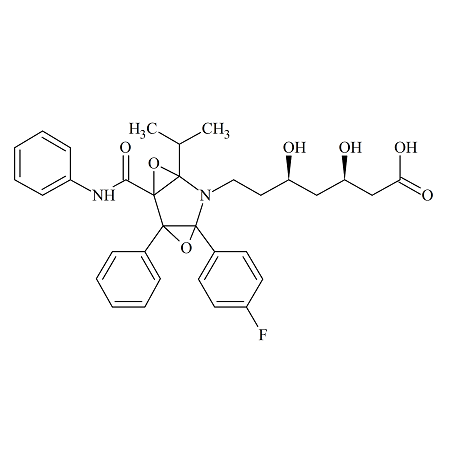
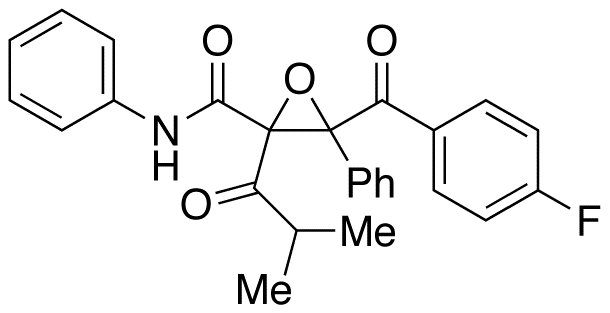
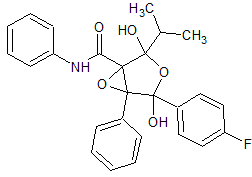
Atorvastatin Diepoxide
- Product Number ATR-16-005
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
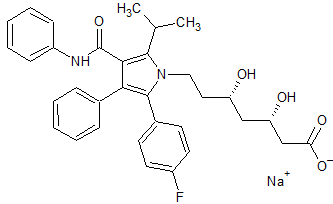
Atorvastatin EP Impurity E(Calcium salt)
- Product Number CT-01110-418
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
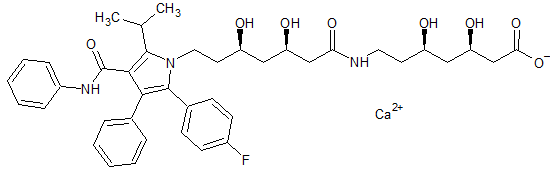
Atorvastatin EP Impurity F(Calcium Salt)
- Product Number CT-01110-420
- Parent Drug Atorvastatin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Atorvastatin Epoxy Tetrahydrofuran Impurity
- Product Number CT-01110-415
- Parent Drug Atorvastatin
- CAS Number 873950-19-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options