Drug Impurities Reference Standards
Showing 251–260 of 1927 results
-
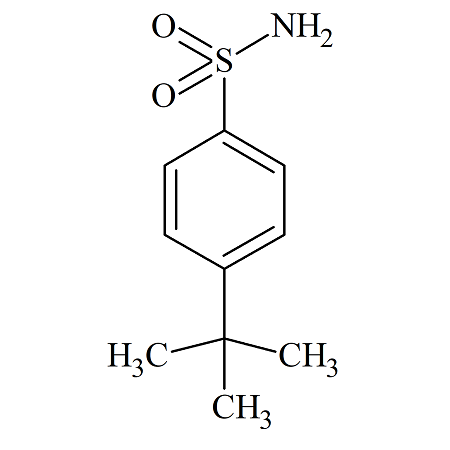
Bortezomib Impurity23
- Product Number CT-01110-164
- Parent Drug Bortezomib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
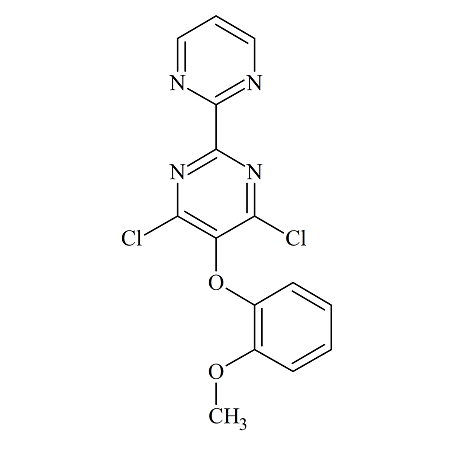
Bosentan USP RC A
- Product Number BOS-12-004
- Parent Drug Bosentan
- CAS Number 150727-06-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Bosentan USP RC B
- Product Number BOS-12-005
- Parent Drug Bosentan
- CAS Number 174227-14-6
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Bosentan USP RC C
- Product Number BOS-12-006
- Parent Drug Bosentan
- CAS Number 1097263-60-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Bosentan USP RC D
- Product Number BOS-12-003
- Parent Drug Bosentan
- CAS Number 150728-13-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Bosentan USP RC E
- Product Number BOS-12-002
- Parent Drug Bosentan
- CAS Number 6292-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
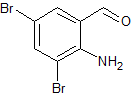
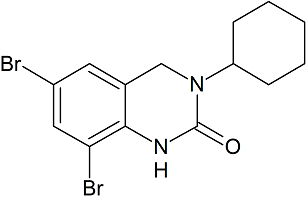
Bromhexine EP Impurity B(Ambroxol EP Impurity E)
- Product Number CT-01110-143
- Parent Drug Bromhexine
- CAS Number 50910-55-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
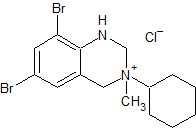
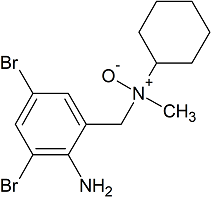
Bromhexine EP Impurity E
- Product Number CT-01110-144
- Parent Drug Bromhexine
- CAS Number 1660957-93-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Bromhexine Impurity 11
- Product Number CT-01110-141
- Parent Drug Bromhexine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 3-4 week(s)See more size options -
Bromhexine Impurity 15
- Product Number CT-01110-142
- Parent Drug Bromhexine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 3-4 week(s)See more size options