Drug Impurities Reference Standards
Showing 271–280 of 1927 results
-
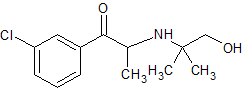
Bupropion Impurity 7
- Product Number CT-01110-536
- Parent Drug Bupropion
- CAS Number 92264-81-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
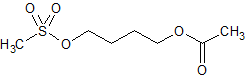
Busulfan Impurity 5
- Product Number CT-01110-813
- Parent Drug Busulfan
- CAS Number 19859-00-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
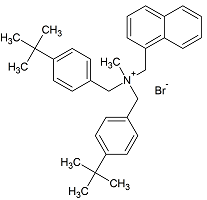
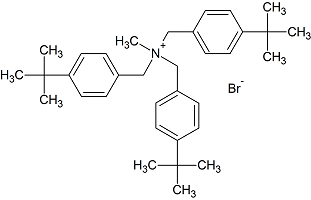
Butenafine Bis(t-butylbenzyl) Bromide
- Product Number B-11029-05
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4-5 week(s)See more size options -
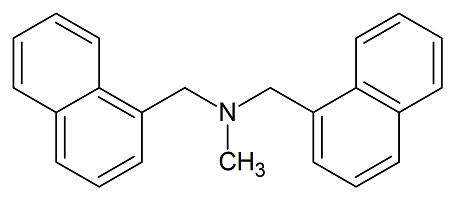
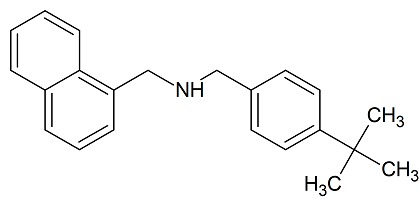
Butenafine Bisnaphthyl Impurity
- Product Number B-11029-06
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4-5 week(s)See more size options -
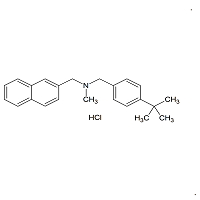
Butenafine Desmethyl Impurity
- Product Number B-11029-07
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4-5 week(s)See more size options -
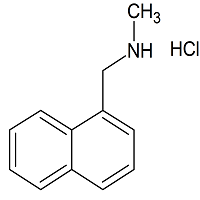
Butenafine Hydrochloride Impurity 1
- Product Number B-11029-01
- Parent Drug Butenafine
- CAS Number 65473-13-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Butenafine Hydrochloride Impurity 3
- Product Number B-11029-02
- Parent Drug Butenafine
- CAS Number 101827-53-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Butenafine Hydrochloride Impurity 4
- Product Number B-11029-03
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
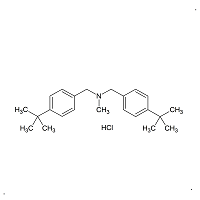
Butenafine Tris(t-butylbenzyl) Bromide
- Product Number B-11029-04
- Parent Drug Butenafine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
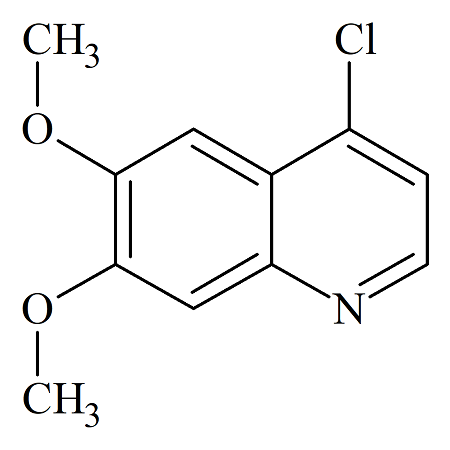
Cabozantinib 4-Chloroquinoline Impurity
- Product Number B-70831-12
- Parent Drug Cabozantinib
- CAS Number 35654-56-9
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options