Drug Impurities Reference Standards
Showing 51–60 of 1927 results
-
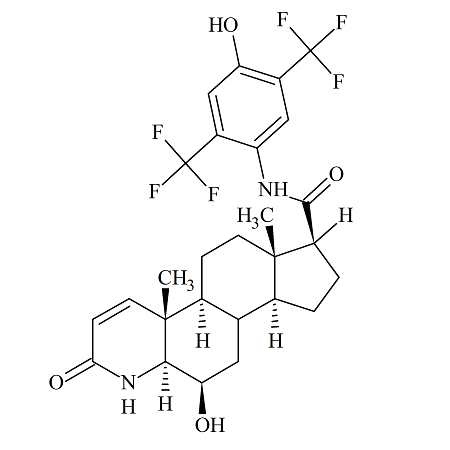
4′,6-Dihydroxy Dutasteride
- Product Number ACA-160819-0023
- Parent Drug Dutasteride
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
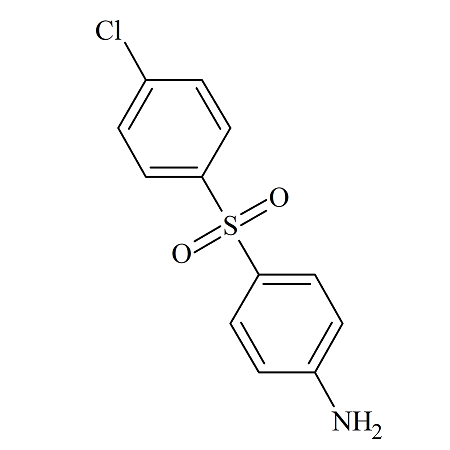
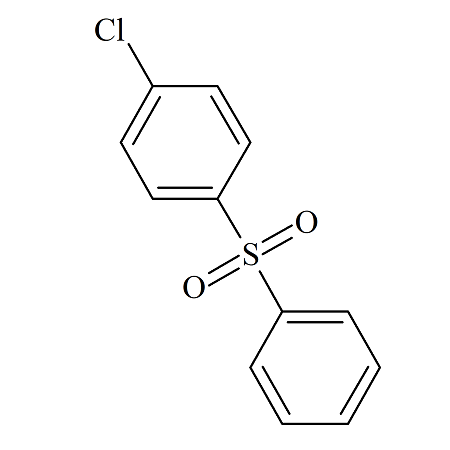
4-Amino-4’-chlorodiphenylsulfone
- Product Number DAP-12-005
- Parent Drug Dapsone
- CAS Number 7146-68-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
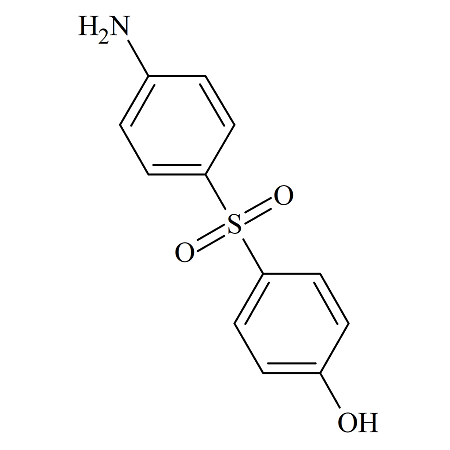
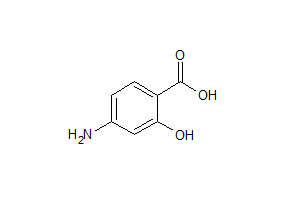
4-Amino-4’-hydroxydiphenylsulfone
- Product Number DAP-12-002
- Parent Drug Dapsone
- CAS Number 25963-47-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
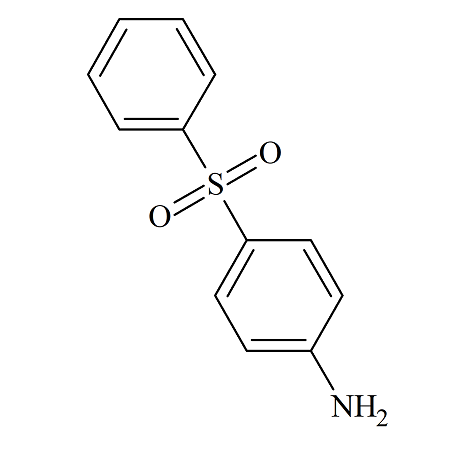
4-Aminodiphenylsulfone
- Product Number DAP-12-004
- Parent Drug Dapsone
- CAS Number 7019-01-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
4-Chlorodiphenylsulfone
- Product Number DAP-12-006
- Parent Drug Dapsone
- CAS Number 80-00-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
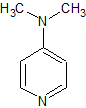
4-Dimethyl Aminopyridine
- Product Number B-71123-0007
- Parent Drug Penciclovir
- CAS Number 1122-58-3
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
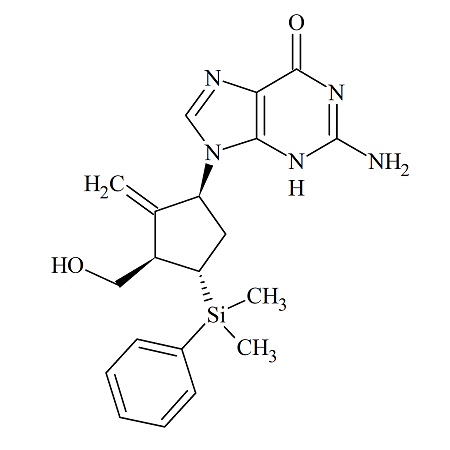
4-Dimethylphenylsilyl-Entecavir
- Product Number ACC-160829-0001
- Parent Drug Entecavir
- CAS Number 701278-07-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
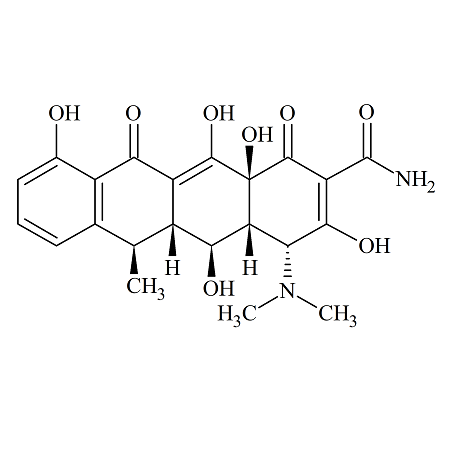
4-Epi Doxycycline
- Product Number ACA-160810-0001
- Parent Drug Doxycycline
- CAS Number 6543-77-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
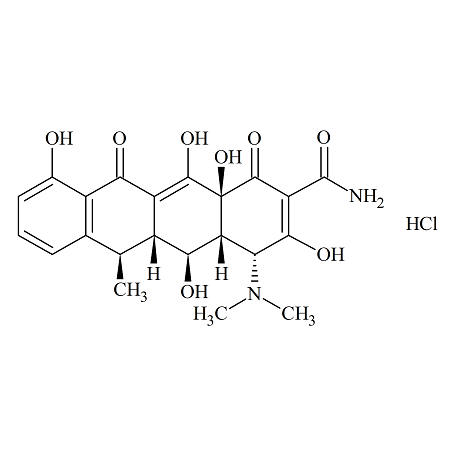
4-Epi Doxycycline Hydrochloride
- Product Number ACA-160824-0002
- Parent Drug Doxycycline
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options