Drug Impurities Reference Standards
Showing 601–610 of 1927 results
-
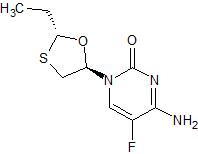
Emtricitabine 5-Epimer
- Product Number CT-01110-555
- Parent Drug Emtricitabine
- CAS Number 145986-26-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
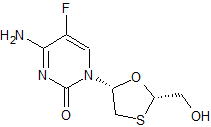
Emtricitabine Enantiomer
- Product Number E-01006-01
- Parent Drug Emtricitabine
- CAS Number 137530-41-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
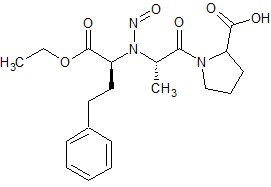
Enalapril N-Nitroso
- Product Number E-10521-01
- Parent Drug Enalapril
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
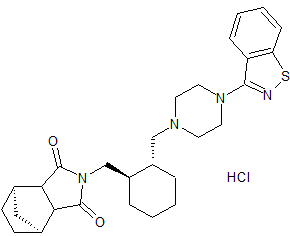
endo-Lurasidone
- Product Number CT-01110-77
- Parent Drug Lurasidone
- CAS Number 1318074-25-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
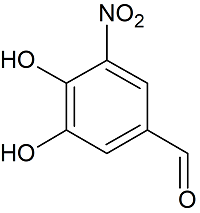
Entecapone Related Compound C
- Product Number E-10901-03
- Parent Drug Entecapone
- CAS Number 116313-85-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
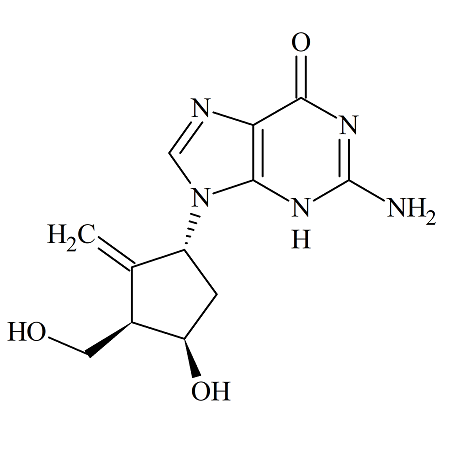
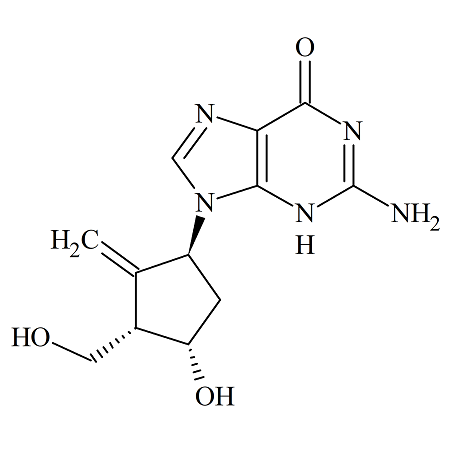
Entecavir (1R, 3R, 4R) Diastereomer
- Product Number ACA-161006-0011
- Parent Drug Entecavir
- CAS Number 1367369-76-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
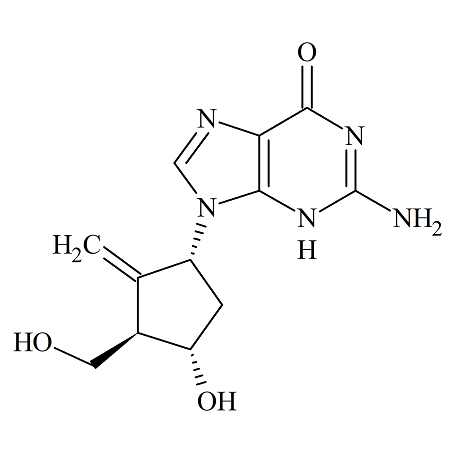
Entecavir (1R,3R,4S) Diastereomer
- Product Number ENT-12-002
- Parent Drug Entecavir
- CAS Number 1367369-78-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
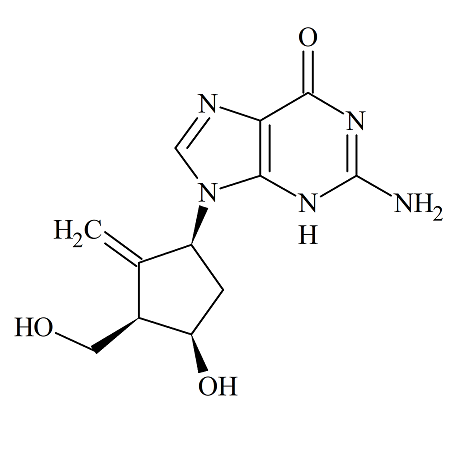
Entecavir (1S,3R,4R) Diastereomer
- Product Number ENT-13-008
- Parent Drug Entecavir
- CAS Number 1367369-80-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Entecavir (1S,3S,4S) Diastereomer
- Product Number ENT-13-007
- Parent Drug Entecavir
- CAS Number 1367369-77-4
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
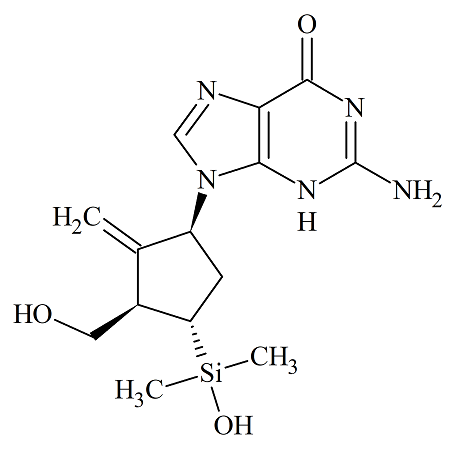
Entecavir 4-Dimethylsilyl USP Impurity
- Product Number ENT-13-002
- Parent Drug Entecavir
- CAS Number 870614-82-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options