Drug Impurities Reference Standards
Showing 631–640 of 1927 results
-
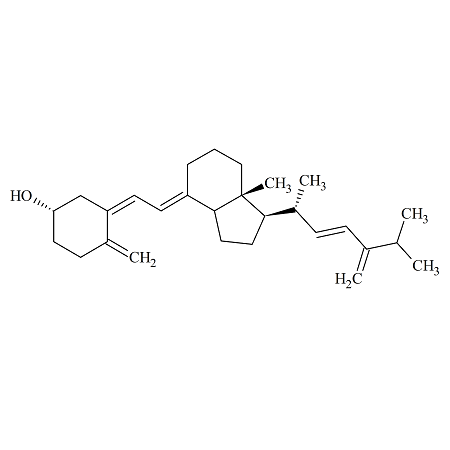
Ergocalciferol Dehydro Impurity
- Product Number ERG-09-001
- Parent Drug Vitamin D2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
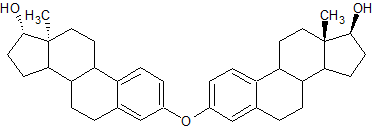
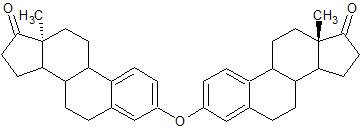
Estradiol 3-3′ Dimer
- Product Number E-90916-02
- Parent Drug Estradiol
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Estrone 3-3′ Dimer
- Product Number E-90917-01
- Parent Drug Estrone
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
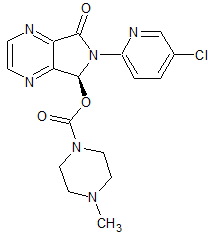
Eszopiclone (S-Zopiclone)
- Product Number CT-01110-570
- Parent Drug Zopiclone
- CAS Number 138729-47-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
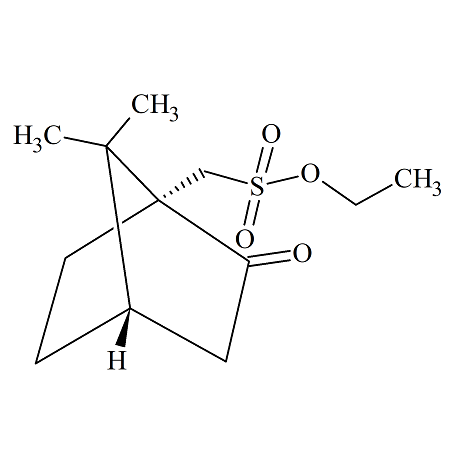
Ethyl (+/-)-10-Camphorsulfonate
- Product Number CAM-09-002
- Parent Drug Camphorsulfonates
- CAS Number 108481-13-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
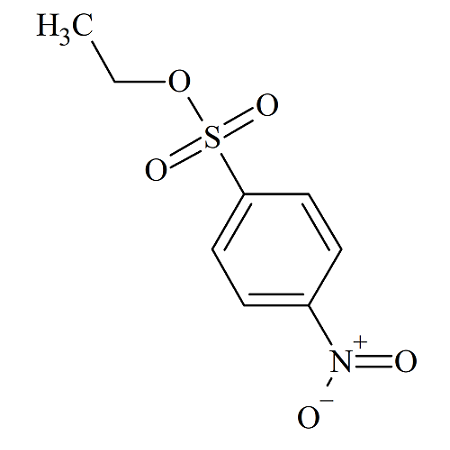
Ethyl 4-Nitrobenzenesulfonate
- Product Number NBS-10-002
- Parent Drug p-Nitrobenzenesulfonates
- CAS Number 15481-55-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
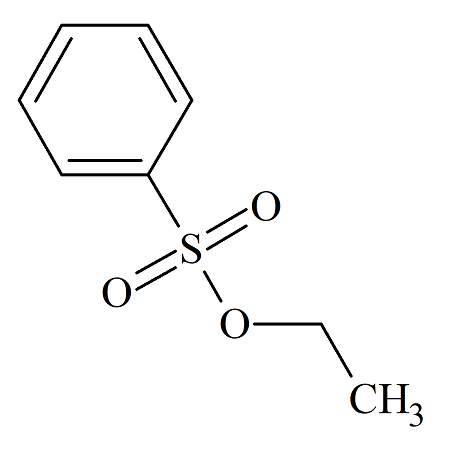
Ethyl Benzenesulfonate
- Product Number BES-08-002
- Parent Drug Benzenesulfonates
- CAS Number 515-46-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
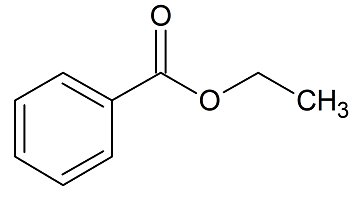
Ethyl Benzoate Certified Reference Standard
- Product Number B-10119-01
- Parent Drug Benzoates
- CAS Number 93-89-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options