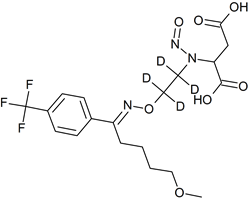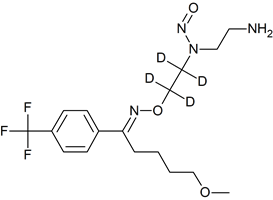Drug Impurities Reference Standards
Showing 701–710 of 1927 results
-
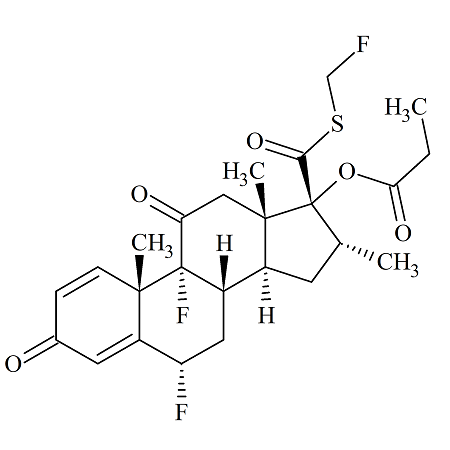
Fluticasone Propionate Disulfide Dimer Impurity
- Product Number FLC-14-003
- Parent Drug Fluticasone
- CAS Number 201812-64-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fluticasone Propionate EP Impurity G
- Product Number CT-01110-901
- Parent Drug Fluticasone
- CAS Number 220589-37-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Fluticasone Propionate Ketone Impurity
- Product Number FLC-14-002
- Parent Drug Fluticasone
- CAS Number 1219174-94-3
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fluticasone Propionate Sulfenic Acid Impurity
- Product Number FLC-14-001
- Parent Drug Fluticasone
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fluvoxamine EP Impurity C-D4 N-nitroso
- Product Number F-40820-03
- Parent Drug Fluvoxamine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fluvoxamine EP Impurity F-D4 N-nitroso
- Product Number F-40820-02
- Parent Drug Fluvoxamine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
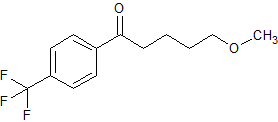
Fluvoxketone
- Product Number F-91003-1
- Parent Drug Fluvoxketone
- CAS Number 61718-80-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
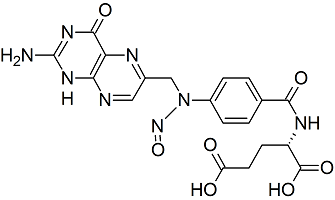
Folic Acid N-Nitroso
- Product Number F-30318-01
- Parent Drug Folic Acid
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
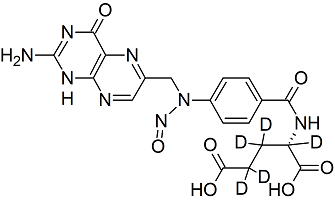
Folic Acid-D5 N-Nitroso
- Product Number F-30201-01
- Parent Drug Folic Acid
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Formaldehyde Certified Reference Standard
- Product Number FCD-20315-01
- Parent Drug Fine Chemicals
- CAS Number 50-00-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options