Drug Impurities Reference Standards
Showing 711–720 of 1927 results
-
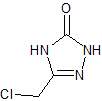
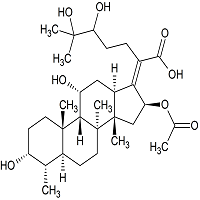
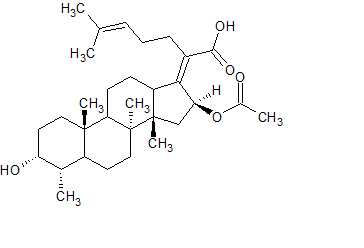
Fosaprepitant Impurity 18
- Product Number CT-01110-490
- Parent Drug Aprepitant
- CAS Number 252742-72-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
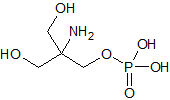
Fosfomycin Trometamol EP Impurity C
- Product Number CT-01110-626
- Parent Drug Fosfomycin
- CAS Number 23001-39-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
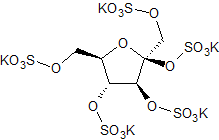
Fructose Pentasulfate Potassium Salt
- Product Number S-90429-02
- Parent Drug Sucralfate
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
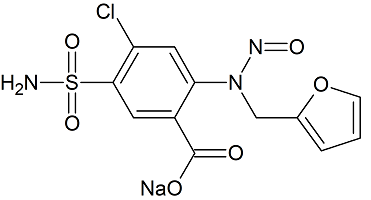
Furosemide N-nitroso Sodium Salt
- Product Number F-30914-01
- Parent Drug Furosemide
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
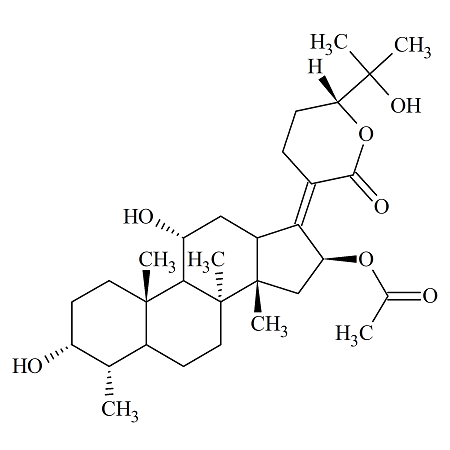
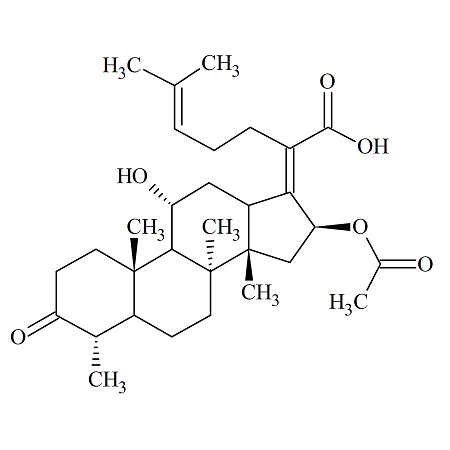
Fusidic Acid EP Impurity A
- Product Number F-10427-01
- Parent Drug Fusidic Acid
- CAS Number 80445-74-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fusidic Acid EP Impurity C
- Product Number FUS-14-005
- Parent Drug Fusidic Acid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
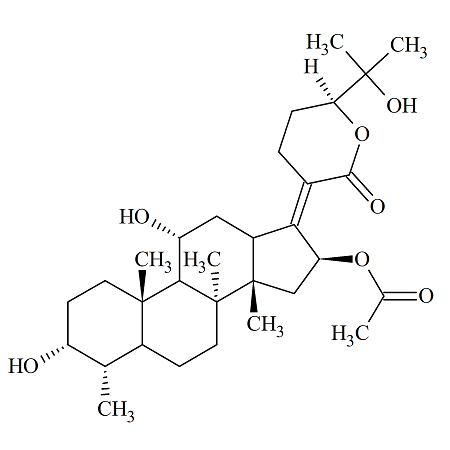
Fusidic Acid EP Impurity D
- Product Number FUS-14-004
- Parent Drug Fusidic Acid
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
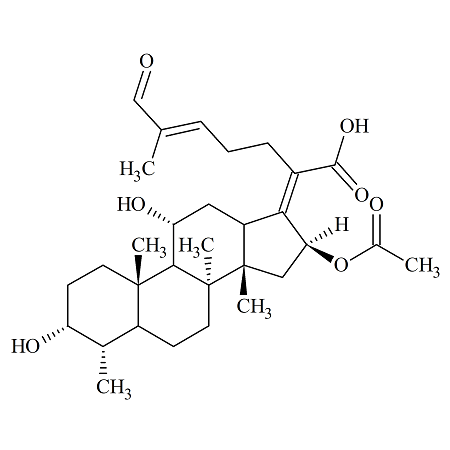
Fusidic Acid EP Impurity F
- Product Number FUS-14-002
- Parent Drug Fusidic Acid
- CAS Number 1415035-94-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fusidic Acid EP Impurity M
- Product Number FUS-14-003
- Parent Drug Fusidic Acid
- CAS Number 1013937-16-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Fusidic Acid EP impurityG
- Product Number ACA-160907-0001
- Parent Drug Fusidic Acid
- CAS Number 4680-37-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options