Drug Impurities Reference Standards
Showing 721–730 of 1927 results
-
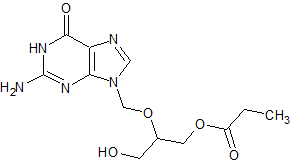
Ganciclovir EP Impurity B
- Product Number CT-01110-72
- Parent Drug Ganciclovir
- CAS Number 194159-18-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
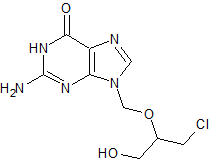
Ganciclovir EP Impurity C
- Product Number CT-01110-73
- Parent Drug Ganciclovir
- CAS Number 108436-36-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
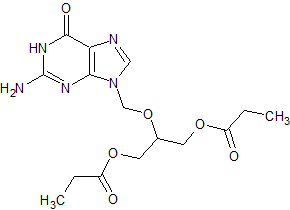
Ganciclovir EP Impurity I
- Product Number CT-01110-74
- Parent Drug Ganciclovir
- CAS Number 86357-20-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
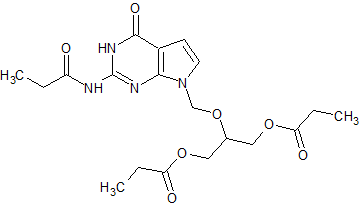
Ganciclovir EP Impurity J
- Product Number CT-01110-71
- Parent Drug Ganciclovir
- CAS Number 177216-32-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
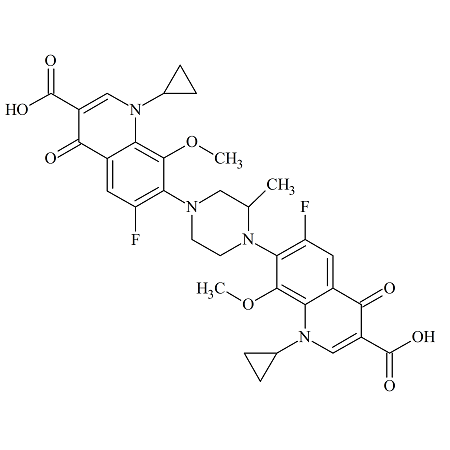
Gatifloxacin Dimer 1
- Product Number ACA-161006-0002
- Parent Drug Gatifloxacin
- CAS Number 1497338-46-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
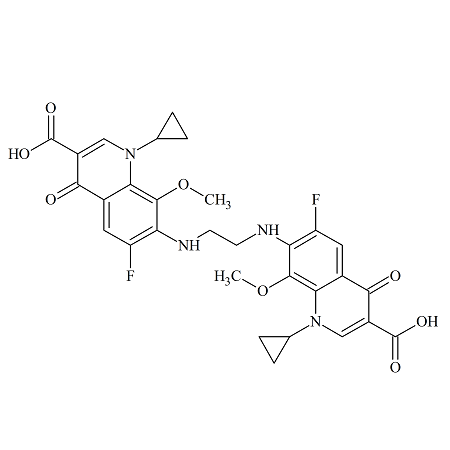
Gatifloxacin Dimer 4
- Product Number ACA-161006-0001
- Parent Drug Gatifloxacin
- CAS Number 1497338-53-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Gatifloxacin Impurity 4
- Product Number CT-01110-599
- Parent Drug Gatifloxacin
- CAS Number 103460-89-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
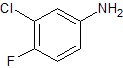
Gefitinib Impurity 11
- Product Number CT-01110-395
- Parent Drug Gefitinib
- CAS Number 367-21-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
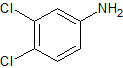
Gefitinib Impurity 13
- Product Number CT-01110-396
- Parent Drug Gefitinib
- CAS Number 95-76-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options