Drug Impurities Reference Standards
Showing 851–860 of 1927 results
-
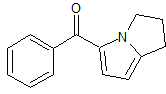
Ketorolac EP Impurity A
- Product Number CT-01110-908
- Parent Drug Ketorolac
- CAS Number 154476-25-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
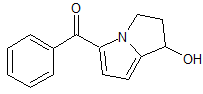
Ketorolac EP Impurity D
- Product Number CT-01110-910
- Parent Drug Ketorolac
- CAS Number 1391053-45-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
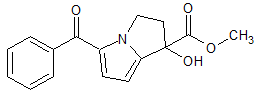
Ketorolac EP Impurity G
- Product Number CT-01110-911
- Parent Drug Ketorolac
- CAS Number 1391051-90-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
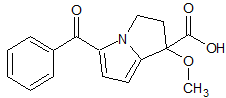
Ketorolac EP Impurity I
- Product Number CT-01110-909
- Parent Drug Ketorolac
- CAS Number 113502-55-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
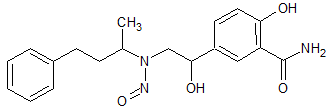
Labetalol N-Nitroso
- Product Number L-10521-01
- Parent Drug Labetalol
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
Lacidipine N-Nitroso
- Product Number L-10521-02
- Parent Drug Lacidipine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
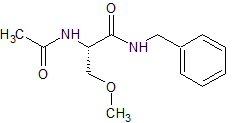
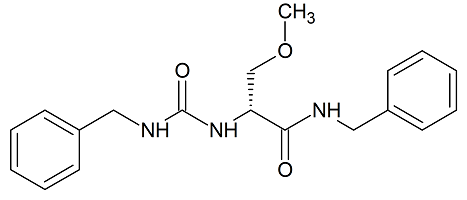
Lacosamide EP Impurity A
- Product Number CT-01110-1013
- Parent Drug Lacosamide
- CAS Number 175481-37-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lacosamide EP Impurity C (R-isomer)
- Product Number CT-01110-1015
- Parent Drug Lacosamide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
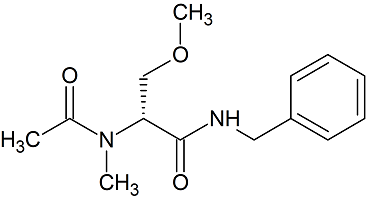
Lacosamide EP Impurity H
- Product Number CT-01110-1012
- Parent Drug Lacosamide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
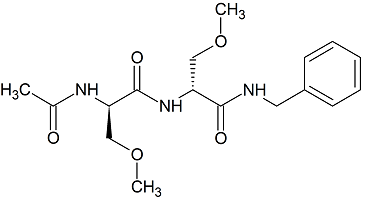
Lacosamide EP Impurity I
- Product Number CT-01110-1014
- Parent Drug Lacosamide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options