Drug Impurities Reference Standards
Showing 891–900 of 1927 results
-
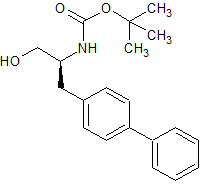
LCZ-696 Impurity 66
- Product Number CT-01110-951
- Parent Drug LCZ-696
- CAS Number 153037-40-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
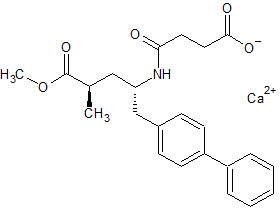
LCZ-696 Impurity 72 calcium salt
- Product Number CT-01110-952
- Parent Drug LCZ-696
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
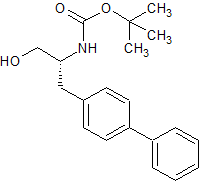
LCZ-696 Intermediate 39
- Product Number CT-01110-947
- Parent Drug LCZ-696
- CAS Number 1426129-50-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
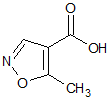
Leflunomide EP Impurity D
- Product Number CT-01110-912
- Parent Drug Leflunomide
- CAS Number 42831-50-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
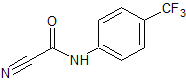
Leflunomide EP Impurity E
- Product Number CT-01110-913
- Parent Drug Leflunomide
- CAS Number 208401-20-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
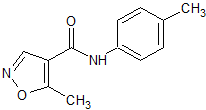
Leflunomide EP Impurity F
- Product Number CT-01110-914
- Parent Drug Leflunomide
- CAS Number 1403564-06-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
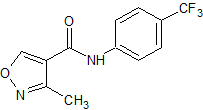
Leflunomide EP Impurity G
- Product Number CT-01110-915
- Parent Drug Leflunomide
- CAS Number 724429-16-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
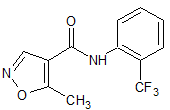
Leflunomide EP Impurity H
- Product Number CT-01110-916
- Parent Drug Leflunomide
- CAS Number 24522-30-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
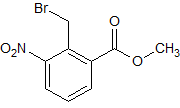
Lenalidomide Impurity 16
- Product Number CT-01110-1059
- Parent Drug Lenalidomide
- CAS Number 98475-07-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
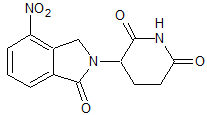
Lenalidomide Impurity 9
- Product Number CT-01110-1060
- Parent Drug Lenalidomide
- CAS Number 827026-45-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options