Drug Impurities Reference Standards
Showing 921–930 of 1927 results
-
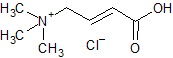
Levocarnitine EP Impurity A(Z/E Mixture)
- Product Number CT-01110-618
- Parent Drug Carnitine
- CAS Number 927-89-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
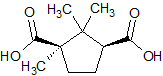
Levocarnitine EP Impurity B
- Product Number CT-01110-617
- Parent Drug Carnitine
- CAS Number 124-83-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
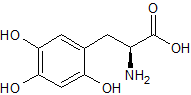
Levodopa EP Impurity A
- Product Number CT-01110-917
- Parent Drug Levodopa
- CAS Number 27244-64-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
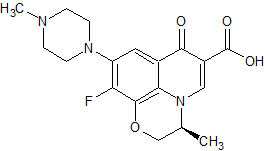
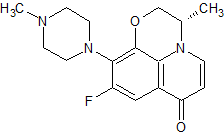
Levofloxacin 9-Piperazinyl Isomer
- Product Number CT-01110-701
- Parent Drug Levofloxacin
- CAS Number 178912-62-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
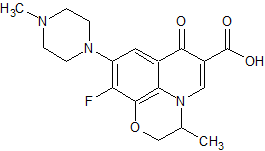
Levofloxacin Descarboxy Impurity(Levofloxacin EP Impurity E)
- Product Number CT-01110-703
- Parent Drug Levofloxacin
- CAS Number 178964-53-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
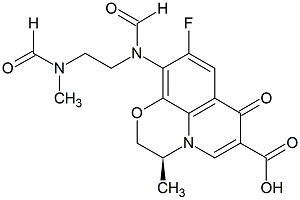
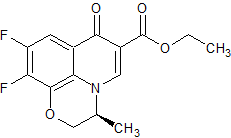
Levofloxacin Desethylene Diformyl Impurity
- Product Number CT-01110-700
- Parent Drug Levofloxacin
- CAS Number 151377-74-1
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: week(s)See more size options -
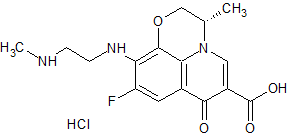
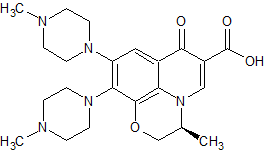
Levofloxacin Diamine Derivative
- Product Number CT-01110-709
- Parent Drug Levofloxacin
- CAS Number 1346603-62-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Levofloxacin Impurity 13
- Product Number CT-01110-702
- Parent Drug Levofloxacin
- CAS Number 197291-75-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Levofloxacin Impurity 26
- Product Number CT-01110-705
- Parent Drug Levofloxacin
- CAS Number 106939-34-8
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Levofloxacin Impurity 32
- Product Number CT-01110-707
- Parent Drug Levofloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options