Drug Impurities Reference Standards
Showing 971–980 of 1927 results
-
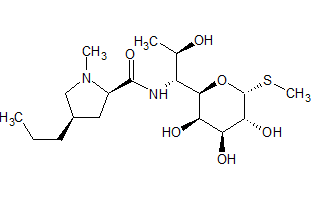
Lincomycin a-Amide Epimer
- Product Number L-90116-01
- Parent Drug Lincomycin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
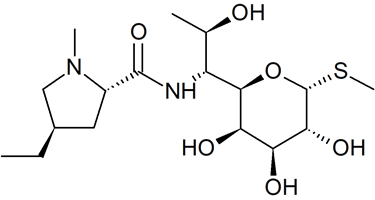
Lincomycin B
- Product Number L-50410-01
- Parent Drug Clindamycin
- CAS Number 2520-24-3
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
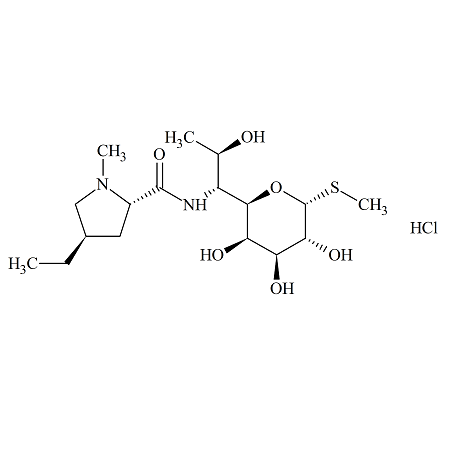
Lincomycin B (as Hydrochloride)
- Product Number LIN-16-001
- Parent Drug Lincomycin
- CAS Number 11021-35-5
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
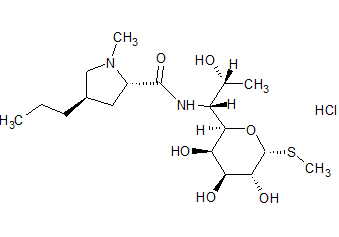
Lincomycin HCl
- Product Number ACC-171213-0001
- Parent Drug Clindamycin
- CAS Number 859-18-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
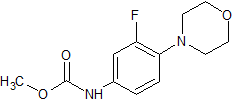
Linezolid Desacetamide Hydroxy Impurity
- Product Number CT-01110-173
- Parent Drug Linezolid
- CAS Number 168828-82-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
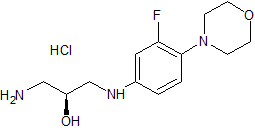
Linezolid Impurity 1
- Product Number CT-01110-166
- Parent Drug Linezolid
- CAS Number 189038-36-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
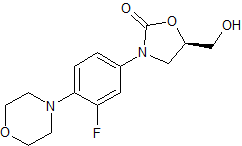
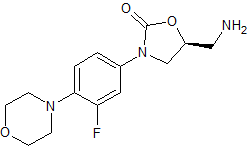
Linezolid Impurity 18
- Product Number CT-01110-167
- Parent Drug Linezolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
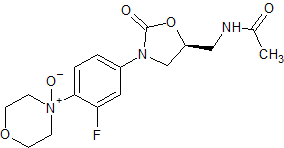
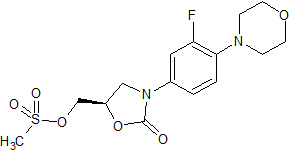
Linezolid Impurity 19
- Product Number CT-01110-168
- Parent Drug Linezolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linezolid Impurity 2
- Product Number CT-01110-169
- Parent Drug Linezolid
- CAS Number 168828-90-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Linezolid Impurity 22
- Product Number CT-01110-170
- Parent Drug Linezolid
- CAS Number 174649-09-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options