Why is control of N-nitrosamine impurities in drugs important?
Many nitrosamines (N-Nitrosamines) are potent genotoxins; some have been classified as possible human carcinogens. Recently several toxic nitrosamines have been found as impurities in human drugs. As a result of this discovery, 2020 FDA guidance mandates control of N-nitrosamine impurities in pharmaceuticals. See our post ‘Control of Nitrosamines in Human Drugs’ for more background information.
Let’s now focus on the structure of N-nitrosamines and implications for their chemical analysis.
Can N-Nitrosamines exist in two different stable forms?
N-nitrosamines substituted with two different alkyl groups can exist in two different stable forms distinguishable by several analytical methods.
Usually, N-nitrosamines are drawn as the structure shown on the left in (Fig.1). However, the zwitterionic resonance structures shown on the right are stable enough to be observed by several analytical methods (Ref. 1).
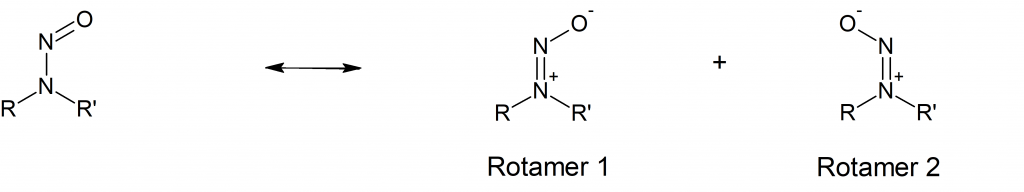
In the two zwitterionic resonance forms the rotation around the nitrogen-nitrogen bond is restricted, leading to the two identifiable rotamers when R and R’ are not the same. The two rotamers may differ in their overall dipole moment and therefore may posses different chemical and physical properties. The relative ratio of the two rotamers of N-nitrosamines depends on their relative stability. Based on analogy to cis/trans (Z/E) isomerism in asymetric alkenes the rotamers are sometimes referred to as cis (Z) or trans (E) forms.
Why do I see two species in NMR of some N-nitrosamines?
N-nitrosamine rotamers are observed by Nuclear Magnetic Resonance (NMR) spectroscopy. For example, the two rotamers of N-nitroso methylethylamine show different chemical shifts (Ref 2): chemical shifts of the methylene protons are 6.4 ppm and 5.8 ppm, for cis and trans forms, respectively, in a ratio of 8 to 2 cis/trans.
Why do I see two peaks when analyzing N-Nitrosamines by HPLC or GC?
High Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) analysis of N-nitrosamines may show two rotamers of the N-nitrosamine as two separate peaks. Whether two peaks are observed depends on the structure of the N-nitrosamine (i.e., symmetric vs. asymmetric) as well as the separation power of the analytical method.
Let’s consider HPLC analysis of seven FDA-identified N-nitrosamine impurities: N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitroso-N-methyl-4-aminobutanoic acid (NMBA), N-nitrosoisopropylethyl amine (NIPEA), N-nitrosomethyl-phenylamine (NMPA), N-nitrosodiisopropylamine (NDIPA) and N-nitrosodibutylamine (NDBA). Four of these compounds, namely NDMA, NDEA, NDIPA and NDBA, are symmetric dialkylamines. These symmetric nitrosamines are expected to give a single peak since the zwitterionic forms are equivalent. However, the chromatograms of NMBA, NIPEA and NMPA may show two peaks resulting from the two stable rotamers. Note that during the chromatographic analysis of these compounds, resolution of the two configurations may not have been achieved. In addition, the minor rotamer may be missed during analysis, and only the major form reported.
At Acanthus Research, we have observed a set of two peaks in the analysis of several N-nitroso derivatives of drug molecules. For example, the HPLC analysis of metopropol N-nitroso showed two peaks at 8.9 min and 9.4 min; the HPLC trace of ramipril N-nitroso gave two peaks at 15.7 min and 17.0 min. The proposed stable rotamers of metopropol N-nitroso and ramipril N-nitroso are shown in Figure 2.
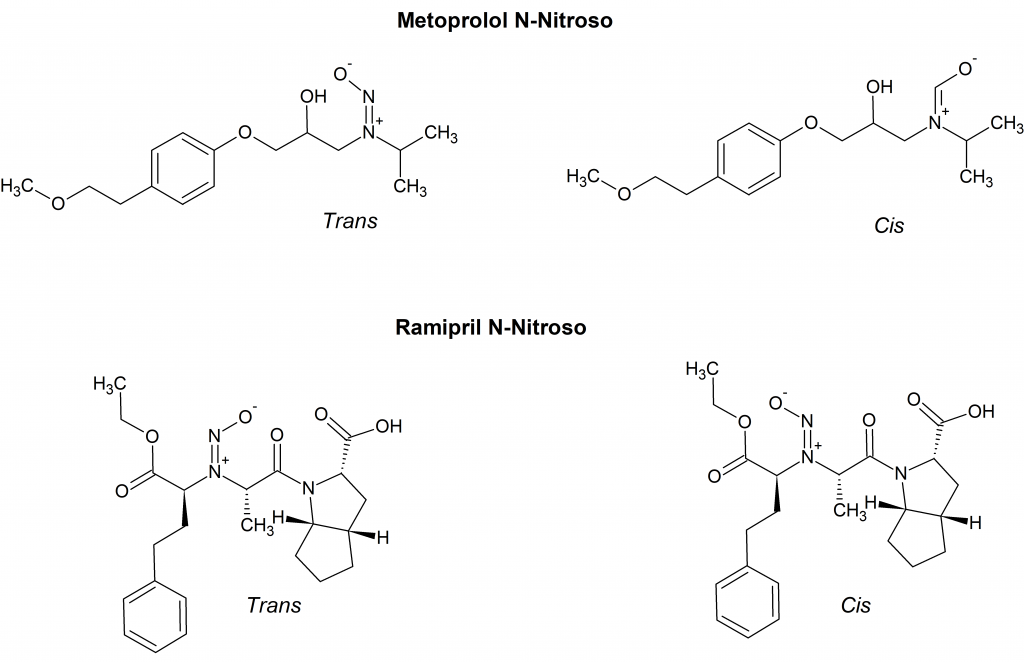
In the case of metoprolol N-nitroso the rotamers ratio according to HPLC analysis is 13.5 to 86.2. The two stable rotamers of ramipril N-nitroso appear to be nearly equivalent with a ratio of 47.4 to 50.5. The HPLC analyses are consistent with NMR characterization.
Other N-nitroso derivatives of API’s that we observed as two separate speaks in HPLC chromatograms are:
N-Desmethyl Clarithromycin N-Nitroso
Rilpivirine N-Nitroso
Enalapril N-Nitroso
Lisinopril N-Nitroso
Perindopril N-Nitroso
What is the take home message on the structure of N-nitrosamines?
In short, it is important to consider the possibility of existence of stable of N-nitrosamine rotamers. Most often the structure has been assumed to consist of a nitrogen-oxygen double bond (N-N=O). However, in asymmetric secondary amines, restricted rotation around the N-N bond, which assumes a double bond character, leads to two different stable rotamers, sometimes called trans and cis isomers. These two forms may be observed by NMR and various chromatographic techniques.
References:
- Jessica C. Beard and Timothy M. Swager, An Organic Chemist’s Guide to N‑Nitrosamines: Their Structure, Reactivity, and Role as Contaminants, J. Org. Chem. 2021, 86, 2037−2057.
- G. J. Karabatsos, R. A. Taller, Structural Studies by Nuclear Magnetic Resonance. IX. Configurations and Conformations of N-Nitrosamines, J. Am. Chem. Soc. 1964, 20, 4373-4378.
