Written By: John Tobin Ph.D.
Quantitative NMR Spectroscopy
What is qNMR?
Quantitative NMR (qNMR) uses nuclear magnetic resonance spectroscopy to quantify chemical species, i.e., determine their concentration in solution. Of course, NMR is known as a powerful structural assignment tool used to establish the identity of chemical species, but it can also be used for accurate quantitative measurement with properly designed experiments. We have found that quantitative NMR is a valuable tool for assay determination, i.e., accurate purity measurements of pharmaceutical reference standards.
How does qNMR work?
Quantitative NMR (qNMR) uses the data from a nuclear magnetic resonance spectrum, namely the area or integral of an NMR peak, to determine the concentration of a molecule in solution. Under selected instrumental conditions, the integral is proportional to the number of nuclei from the corresponding signal and the substrate concentration. This is true for all molecules. Thus, qNMR has advantages over other quantitative techniques such as UV-Visible detection in which responses tend to be compound specific. Proton NMR experiments are most common but other nuclei such as phosphorus (31P) or fluorine (19F) can also be used in qNMR. It is necessary that the compound has an NMR-active nuclitide, which is specific to the analyte of interest. Quantitation can be achieved by making use of either an external or an internal calibration standard.
Quantitative NMR has been used to simplify the overall purity determination of reference materials. qNMR can be an alternative to the mass balance method by measuring the amount of analyte directly. The qNMR purity determination method has been adopted by the pharmacopeia such as described in USP General Chapter <761>.
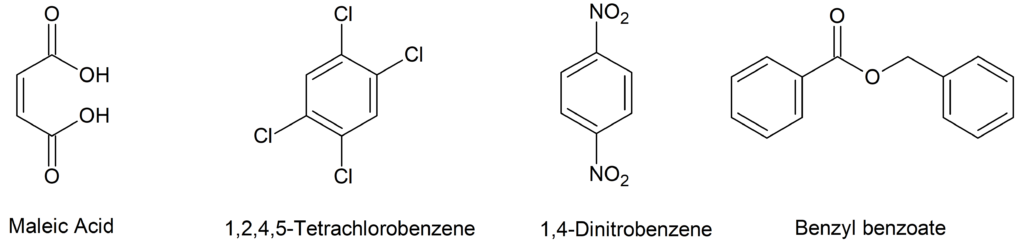
How to Choose an Internal Standard for qNMR?
For use in a quantitative NMR, an internal calibration standard is chosen that is appropriate to the assay determination. The standard’s NMR signal used for quantitation must not interfere by overlapping with signals from the analyte. The internal standard must also possess adequate solubility, must not react with the analyte, and should also be of high purity. Example internal standards (IS) for proton NMR are maleic acid, 1,2,4,5-tetrachlorobenzene, 1,4-dinitrobenzene, or benzyl benzoate. A summary of their NMR characteristics is given in table below.
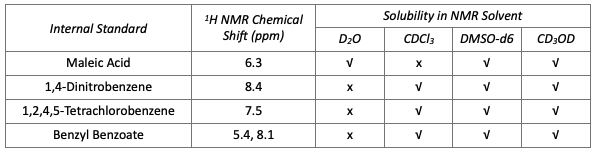
How to Calculate % Assay using qNMR
In an NMR experiment used to assay the analyte, accurate weights of sample and of internal standard are used. In addition, key NMR parameters should be considered, i.e., number of scans chosen for sensitivity, and the use of relaxation delay to ensure signals have fully relaxed between pulses. It is also recommended to employ automatic integration and to use replicate sample preparation for precision assessment.
Calculation of percent assay from the qNMR experiment is done using the following equation:
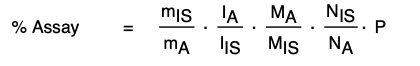

What does the qNMR Assay of a Reference Standard Look Like?
We have used qNMR to determine purity in many reference standard products from Acanthus Research Inc. One example is the drug impurity reference standard: Clindamycin 2-phosphate sulfoxide isomer A (CLI-15-005-A), which is a related compound of clindamycin phosphate active ingredient. Determination of purity directly by qNMR is sought with this standard. The internal standard chosen for the assay is maleic acid. As shown in the NMR spectrum of a mixture of clindamycin 2-phosphate sulfoxide isomer A and maleic acid, the IS signal at 6.3 ppm does not overlap with the analyte signals. The methyl group signal at 0.87 ppm was used in the assay. Triplicate samples of clindamycin 2-phosphate sulfoxide isomer A and internal standard, completely dissolved in DMSO-d6, were prepared. qNMR results are summarized below.
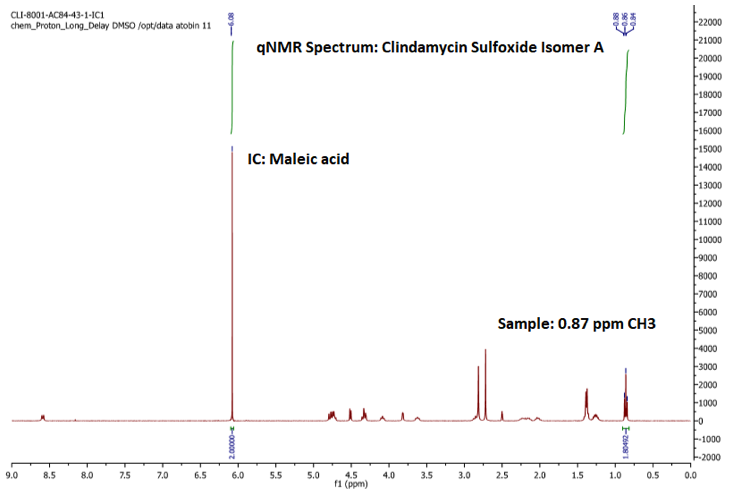
Quantitative NMR Assay of Clindamycin 2-Phosphate Sulfoxide Isomer A in DMSO-d6.
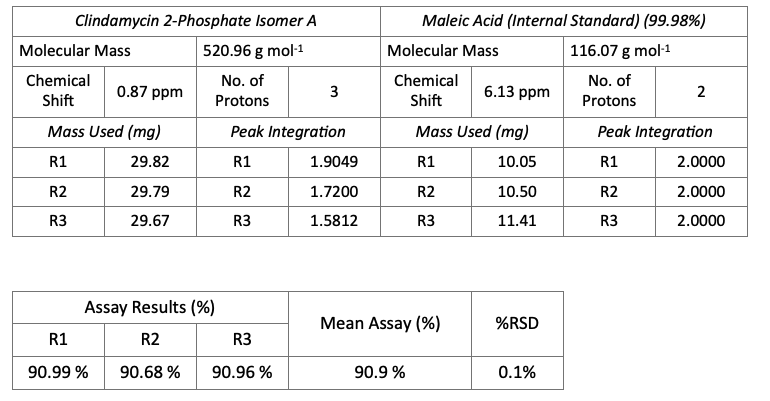
The % assay results for clindamycin 2-phosphate sulfoxide isomer A, shown above, shows good precision with % RSD of 0.1% for the triplicate determinations. The mean % assay value of 90.9% is used as the assigned purity. This result is in consistent with the fact that water is present at about 10% in this standard as shown by thermogravimetric analysis.
The NMR analysis of clindamycin 2-phosphate sulfoxide isomer A demonstrates the utility of quantitative NMR for purity determination in a reference standard.
What are the advantages of qNMR over HPLC?
Quantitative NMR has several advantages over HPLC analysis for purity determination. The main advantage is that qNMR offers a direct measurement of purity. Chromatographic purity by HPLC may overestimate the overall purity of a sample by not detecting all species such as residual solvents or water. Furthermore, qNMR avoids dependency of analyte versus impurity responses often observed in HPLC. In addition, HPLC-UV detection cannot be applied to substances that lack chromophores.
What is the future of qNMR?
Interest in quantitative NMR for analysis is growing as more users in industry recognize that this analytical method has great utility and ease of use. During pharma R&D, chromatographic techniques such as HPLC and GC are widely used analytical techniques. However, their method development is time consuming. Using qNMR can lead to efficiencies by reducing preparation and analysis times. qNMR is also eco-friendly as it reduces solvent use and waste generation. It appears that qNMR analysis will expand its role in the pharmaceutical and related industries.

Other industry papers on qNMR:
University of Oxford: Quantitative NMR Spectroscopy: View Paper
Trinity College Dublin: Quantitative NMR Spectroscopy for the Analysis of Fuels: View Paper