The Promise of mRNA Vaccines
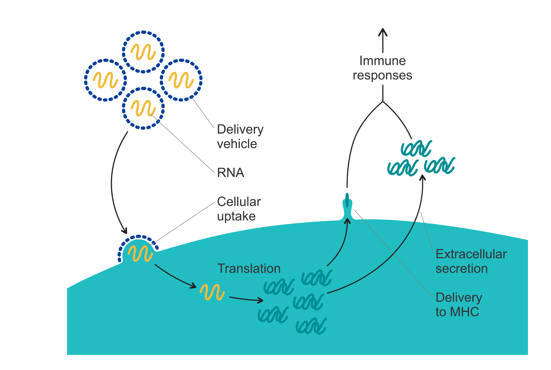
Messenger ribonucleic acid (mRNA) vaccines promise to be more effective and safer than the traditional live-attenuated pathogen and subunit vaccines. They are also easier to develop and potentially safer than DNA vaccines which pose a very low but real risk of incorporation into the cell’s genetic material.
For the mRNA vaccine to be effective, the oligonucleotide must be delivered to the cytosol. This has been problematic for a long time because naked mRNA is quickly degraded by ubiquitous ribonucleases. Among many solutions that have emerged over the years, nonviral vectors and lipid nanoparticles (LNPs) in particular, seem to be the most promising. BioNTech/Pfizer and Moderna COVID-19 vaccines are the first mRNA vaccines approved for use in humans that utilize LNP vector technology.
Design of LNPs for mRNA Vaccines
Suitably designed LNPs should bind and encapsulate mRNA thus protecting it against ribonucleases. The vesicles have to be targeted to the negatively charged cell membrane, undergo endocytosis, and then unload the entrapped oligonucleotide from the endosome into the cytosol making mRNA available for translation (see fig 1). In the COVID-19 vaccines, LNPs contain four different components: an ionizable cationic lipid, a neutral phospholipid, PEG-conjugated lipid, and cholesterol. Phospholipids and cholesterol are incorporated into LNPs to help stabilize the vesicular structure. Phospholipids can also facilitate the disruption of endosomes by virtue of the negative charge of the phosphate group or by enabling the transition from lamellar to hexagonal phase in the endosome. Cholesterol is essential for the transfection of cells. PEG-lipid is added to control particle size and prevent aggregation upon storage.
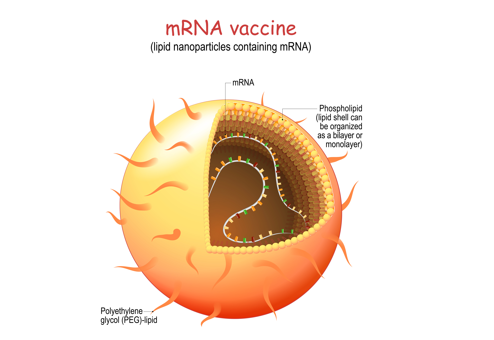
LNPs may be loaded with adjuvants, or they may themselves have an adjuvant effect. Many lipids activate the immune system and induce transient local inflammation which is necessary for vaccine efficacy. The surface of the vesicles may be ‘decorated’ with molecules increasing LNPs affinity to certain cell types: for example, carbohydrates facilitate binding to dendritic cells through specific receptors.
A significant and yet unsolved problem with current mRNA vaccines is their storage stability. Low temperatures are currently required to preserve mRNA integrity. Vaccine stability at 2–8 °C is highly desired. Exposure of mRNA to water seems to be the main reason for RNA instability. Interaction of the cationic lipid with negatively charged mRNA may have a stabilizing effect on oligonucleotide by shielding it from water and by modulating the pH inside the nanoparticle.
Because of the great promise of LNPs in the development of mRNA vaccines, the search for novel lipids and lipid-like materials has greatly intensified in recent years. Improved lipids, derivatized lipids, and lipoids have been developed and used to construct NLPs best able to perform the many functions of an effective mRNA vector. Customization of lipid properties through structure modification, the introduction of unnatural fatty acids, PEG-ylation, or the use of customized combinations of various fatty acids is an important tool in turning their fitness for use in a specific application.
Custom Synthesis of Lipids at Acanthus Research Inc.
At Acanthus Research Inc. and Acanthus Pharma Services Inc., we offer a range of standard lipid products. Some examples are listed below. We can also undertake the synthesis of custom-designed lipids or lipid components to support your developmental and commercial products, simply send a request for quotation.
LIP-21-015 – 1,3-DPPE
LIP-21-001 – DLPA Sodium
LIP-21-016 – DLPC
LIP-21-009 – DLPE
LIP-21-005 – DLPG Sodium
LIP-21-002 – DMPA Sodium
LIP-21-017 – DMPC
LIP-21-010 – DMPE
LIP-21-006 – DMPG Sodium
LIP-21-020 – DPePC Chloride
Connect with us now, send a REQUEST FOR QUOTATION
