Drug Impurities Reference Standards
Showing 1811–1820 of 1927 results
-
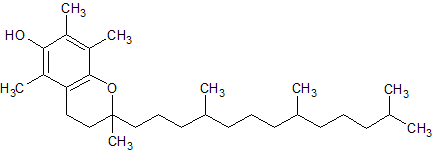
Tocopherol Impurity 5
- Product Number CT-01110-519
- Parent Drug Tocopherol
- CAS Number 10191-41-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
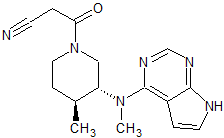
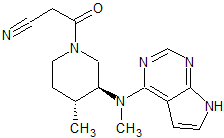
Tofacitinib Impurity 1
- Product Number CT-01110-225
- Parent Drug Tofacitinib
- CAS Number 1092578-46-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
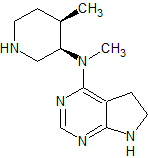
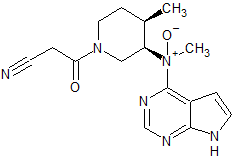
Tofacitinib Impurity 13
- Product Number CT-01110-226
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
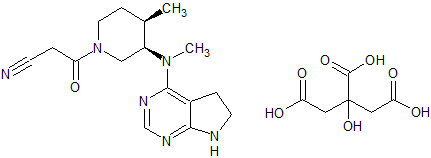
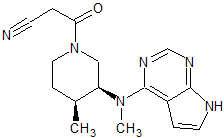
Tofacitinib Impurity 14
- Product Number CT-01110-227
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
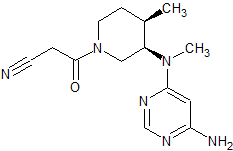
Tofacitinib Impurity 2
- Product Number CT-01110-228
- Parent Drug Tofacitinib
- CAS Number 1092578-48-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
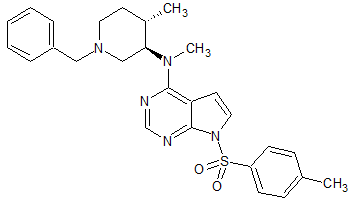
Tofacitinib Impurity 27
- Product Number CT-01110-229
- Parent Drug Tofacitinib
- CAS Number 2028267-73-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 3
- Product Number CT-01110-230
- Parent Drug Tofacitinib
- CAS Number 1092578-47-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
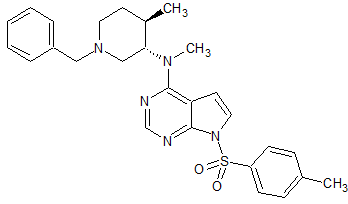
Tofacitinib Impurity 33
- Product Number CT-01110-231
- Parent Drug Tofacitinib
- CAS Number 1640971-60-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 35
- Product Number CT-01110-232
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 36
- Product Number CT-01110-233
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options