Drug Impurities Reference Standards
Showing 1821–1830 of 1927 results
-
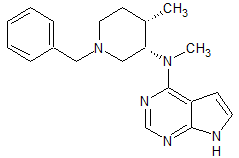
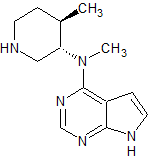
Tofacitinib Impurity 37
- Product Number CT-01110-234
- Parent Drug Tofacitinib
- CAS Number 1252883-90-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
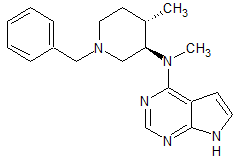
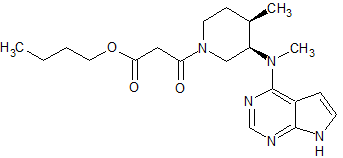
Tofacitinib Impurity 38
- Product Number CT-01110-235
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
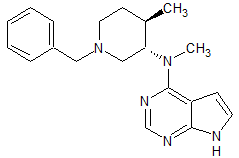
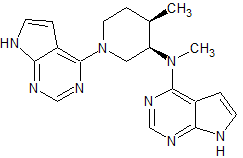
Tofacitinib Impurity 39
- Product Number CT-01110-236
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
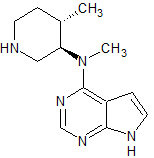
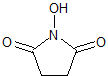
Tofacitinib Impurity 41
- Product Number CT-01110-237
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 42
- Product Number CT-01110-238
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 5
- Product Number CT-01110-239
- Parent Drug Tofacitinib
- CAS Number 1616760-97-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 50
- Product Number CT-01110-240
- Parent Drug Tofacitinib
- CAS Number 2227199-31-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 51
- Product Number CT-01110-241
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 54
- Product Number CT-01110-242
- Parent Drug Tofacitinib
- CAS Number 6066-82-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 6
- Product Number CT-01110-243
- Parent Drug Tofacitinib
- CAS Number 477600-74-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options