Drug Impurities Reference Standards
Showing 1871–1880 of 1927 results
-
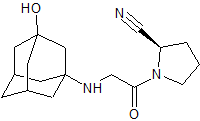
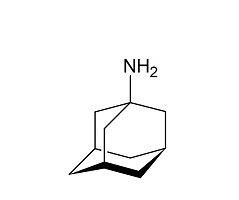
Vildagliptin (2R)-Isomer
- Product Number CT-01110-345
- Parent Drug Vildagliptin
- CAS Number 1036959-27-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
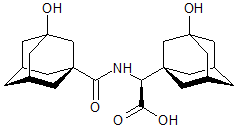
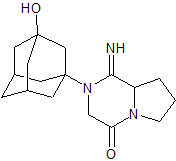
Vildagliptin Impurity 23
- Product Number CT-01110-346
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
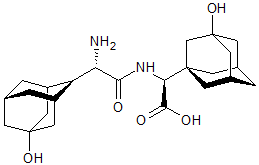
Vildagliptin Impurity 24
- Product Number CT-01110-347
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
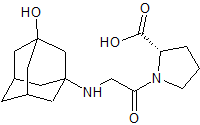
Vildagliptin Impurity 3
- Product Number CT-01110-348
- Parent Drug Vildagliptin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vildagliptin Impurity 31
- Product Number CT-01110-349
- Parent Drug Vildagliptin
- CAS Number 768-94-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vildagliptin Impurity 5
- Product Number CT-01110-350
- Parent Drug Vildagliptin
- CAS Number 1789703-37-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
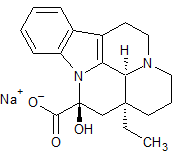
Vinpocetine Impurity 12
- Product Number CT-01110-1036
- Parent Drug Vinpocetine
- CAS Number 59413-21-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
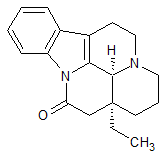
Vinpocetine Impurity 13
- Product Number CT-01110-1037
- Parent Drug Vinpocetine
- CAS Number 4880-88-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
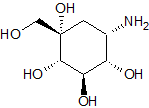
Voglibose Impurity 3
- Product Number CT-01110-982
- Parent Drug Voglibose
- CAS Number 83465-22-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
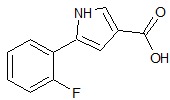
Vonoprazan Fumarate Impurity 17
- Product Number CT-01110-48
- Parent Drug Vonoprazan
- CAS Number 1883595-38-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options