Written By: John Tobin Ph.D.
What are the uses of stable isotope labelled standards?
Stable isotope labelled (SIL) standards are compounds in which several atoms within the molecule are replaced by their stable (non-radioactive) isotopes such as for example deuterium (2H or D), 13C or 15N. The chemical behaviour of the labelled molecule is expected to be very similar to the unlabeled version. Higher mass afforded by the isotopes make the SIL analogs ideal internal standards for chromatographic methods using mass spectroscopic detection. SIL standards are therefore very often used in bioanalytical methods in pharmacokinetics and metabolism studies (Ref 1,2). The use of SIL analogs, when compared to the use of structurally related compounds as internal standards, have been widely shown to reduce effect from matrix and give reproducible and accurate recoveries in LC-MS/MS assays.
What are examples of SIL internal standards?
Below are examples of SIL drug substances and SIL drug metabolites:
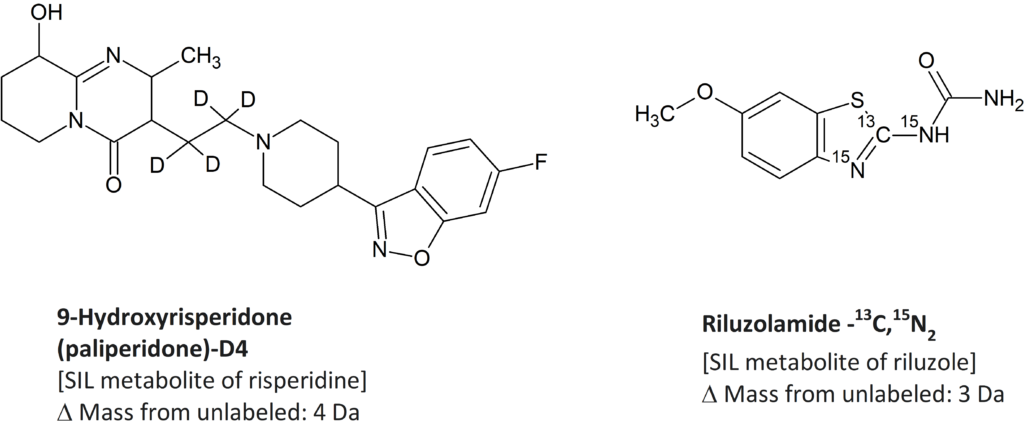
SIL Drug Substances
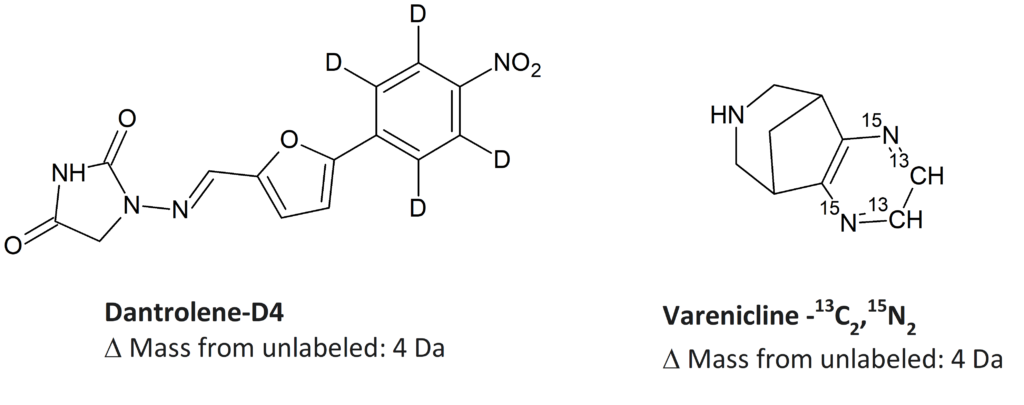
SIL Drug Metabolites
What makes a good SIL internal standard?
Several factors impact the usability of a SIL internal standard: stability of the label, suitable mass difference from the analyte, and non-detectable level of non-labelled species. Sometimes positioning of the label on a specific fragment of the molecule makes the standard much more suitable for LC/MS-MS analysis.
Stability of label require positioning on non-exchangeable sites
Loss or replacement of deuterium by chemical exchange with protons from solvent or from the components of matrix such as blood or plasma, affects the utility of the SIL internal standards. It is therefore important that isotope labels are positioned where exchange is not likely to occur. Of course, deuterium labels should not be placed on heteroatoms such as oxygen (in alcohols, phenols, carboxylic acids, etc.) or nitrogen in amines. In addition, under certain conditions, proton/deuterium exchange may occur if deuterium labels are situated on a carbon adjacent to a carbonyl group, or in some aromatic positions. It is advantageous to use 13C and 15N isotopes as they are not able to exchange. However, deuterium labelling is commonly used due to cost factors.
Suitable Mass Difference
A mass difference between the analyte and the SIL internal standard must be suitable for the analytical application so there are no spectral lines overlap. Generally, with small drug molecules (e.g., less than 1,000 mass units) a difference of three or more mass units is required.
Low Levels of Unlabeled Species
It is very important that SIL internal standard be free of unlabeled species. Ideally, the level of the unlabeled molecule should be undetectable or at least at a level that will not cause interference.
Labelled Positioning on Fragment of Interest
If the mass spectrometry method makes use of the detection of a molecular fragment for quantitative analysis, the labelled site needs to be on the portion of the molecule that is being quantitated. This will make the internal standard differ from the analyte by the desired mass unit.
How is isotope label incorporated into the standard?
There are two methods to introduce isotope labels into a molecule of interest: hydrogen/deuterium exchange and chemical synthesis with isotope-containing building blocks.
Hydrogen/Deuterium exchange
The incorporation of deuterium labels into molecules of interest can be done by hydrogen/deuterium exchange processes (Ref 3). This method is limited to producing deuterium-labelled standards. Hydrogen atoms attached to carbon atoms, not usually exchangeable, can be exchanged under certain conditions such as acid or base catalysis or by use of metal catalysts, and using an excess of deuterated solvent (e.g., deuterium oxide, deuterated methanol, etc.). For example, H/D exchange through means of keto/enol equilibrium allows the introduction of deuterium at alpha position to the carbonyl group by base-catalysis in D2O solution.
Complete synthesis with isotope-containing building blocks
Alternatively, SIL standards can be made by employing classical synthesis, i.e., de novo, using isotopically substituted building blocks. A synthetic route is designed to construct the target molecule so that the stable isotopes are built-in.
For example, for the synthesis of riluzolamide-13C,15N2, the building block, urea-13C,15N2 is used to provide isotope labels. When the benzothiazole ring is constructed with labelled urea (urea-13C,15N2), this molecule will have the same structure as riluzolamide but possess a higher mass (+3 mass units) resulting from the isotopic substitution.
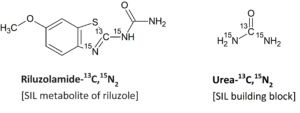
In this synthesized riluzolamide-13C,15N2, the presence of unlabeled species arises only from the isotopic composition of the building block. If the reagent, urea-13C,15N2, has high isotopic incorporation of 13C and 15N, no unlabeled species results in the final product.
How do the approaches to incorporate isotopes into standards compare?
A general comparison of the two presented above methods to produce SIL standards is presented below.
Comparison of Approaches for Incorporation of Label
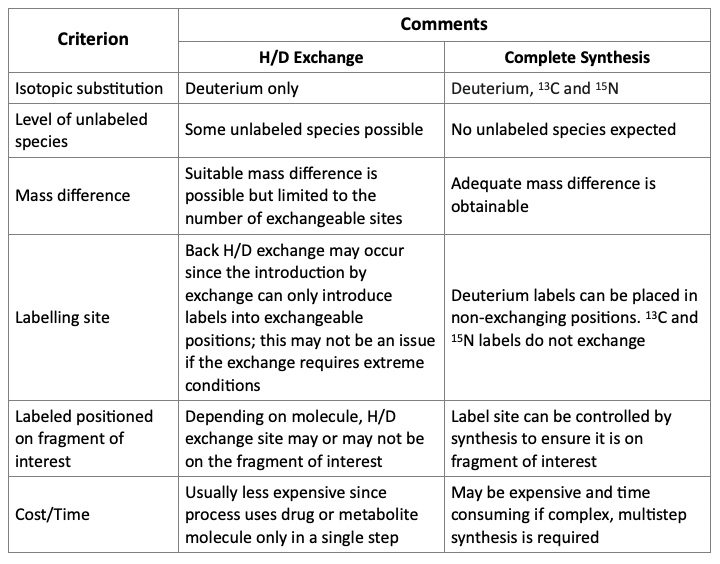
As shown above, the method used to incorporate isotope labels may determine whether a suitable internal standard is produced. Hydrogen/deuterium exchange processes may be the simpler approach. However, complete synthesis offers more flexibility in the position, type, and a number of isotopic substitutions.
References:
- Aimin Tan and Kayode Awaiye, Chapter 17, “Use of Internal Standards in LC/MS Bioanalysis from Handbook of LC‐MS Bioanalysis: Best Practices, Experimental Protocols, and Regulations” Editors: Book Editor(s): Wenkui Li, Jie Zhang, Francis L.S. Tse, 2013.
- Nageswara Rao Reddy, “Stable Labeled Isotopes as Internal Standards: A Critical Review,” Modern Applications in Pharmacy & Pharmacology, 1(2),2017.
- Paulina Grocholska and Remigiusz Bachor, “Review: Trends in the Hydrogen−Deuterium Exchange at the Carbon Centers. Preparation of Internal Standards for Quantitative Analysis by LC-MS Molecules.” 2021, 26, 2989.