Drug Impurities Reference Standards
Showing 271–280 of 1775 results
-
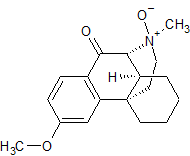
Dextromethorphan N-Oxide
- Product Number CT-01110-459
- Parent Drug Dextromethorphan
- CAS Number 1177494-18-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Amiodarone EP Impurity G(HCl)
- Product Number CT-01110-779
- Parent Drug Amiodarone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
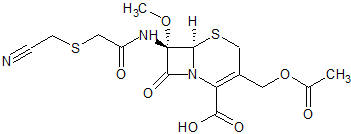
Cefmetazole Impurity 1
- Product Number CT-01110-860
- Parent Drug Cefmetazole
- CAS Number 56796-16-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
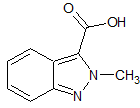
Dasatinib Impurity 3
- Product Number CT-01110-892
- Parent Drug Dasatinib
- CAS Number 910297-51-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
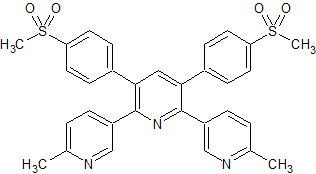
Etoricoxib Dimer Impurity
- Product Number E-10303-01
- Parent Drug Etoricoxib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
Granisetron EP Impurity G
- Product Number CT-01110-1092
- Parent Drug Granisetron
- CAS Number 34252-44-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
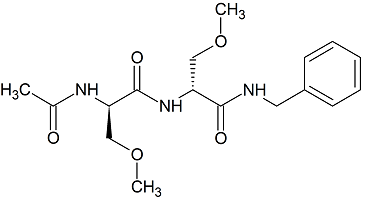
Lacosamide EP Impurity H
- Product Number CT-01110-1012
- Parent Drug Lacosamide
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
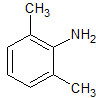
Lidocaine EP Impurity A
- Product Number CT-01110-920
- Parent Drug Lidocaine
- CAS Number 87-62-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
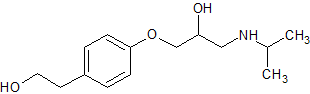
Metoprolol EP Impurity H
- Product Number CT-01110-696
- Parent Drug Metoprolol
- CAS Number 62572-94-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
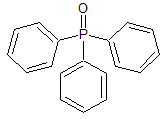
Orlistat Impurity 5
- Product Number CT-01110-845
- Parent Drug Orlistat I
- CAS Number 791-28-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options