Drug Impurities Reference Standards
Showing 21–30 of 1775 results
-
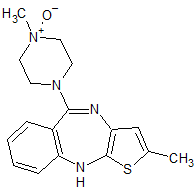
Olanzapine Related Compound C
- Product Number O-10208-02
- Parent Drug Olanzapine
- CAS Number 174794-02-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
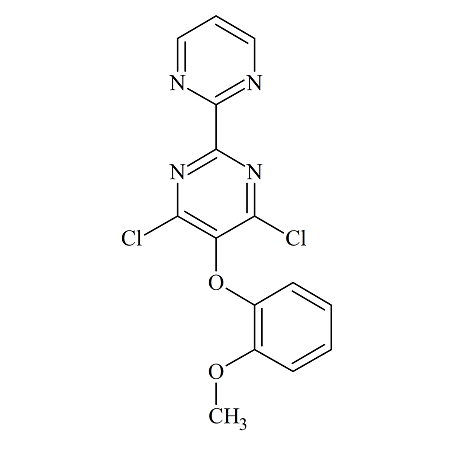
Bosentan USP RC E
- Product Number BOS-12-002
- Parent Drug Bosentan
- CAS Number 6292-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
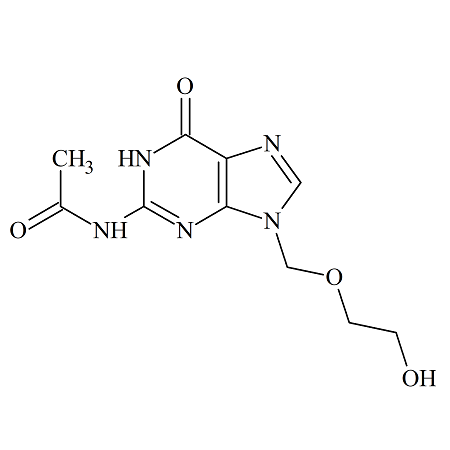
Acyclovir Impurity F
- Product Number ACA-161005-0003
- Parent Drug Acyclovir
- CAS Number 110104-37-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Bosentan USP RC D
- Product Number BOS-12-003
- Parent Drug Bosentan
- CAS Number 150728-13-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
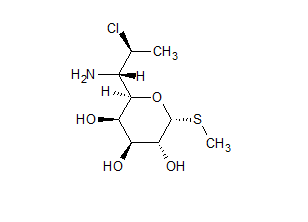
Methyl 1-Thiolincosaminide
- Product Number ACB-161228-0003
- Parent Drug Clindamycin
- CAS Number 14810-93-6
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
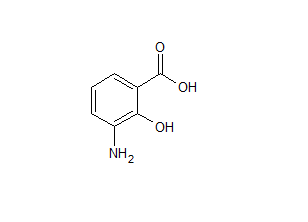
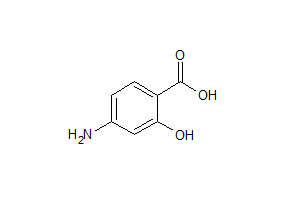
Mesalazine EP Impurity L
- Product Number ACA-161220-0008
- Parent Drug Mesalamine
- CAS Number 118-91-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
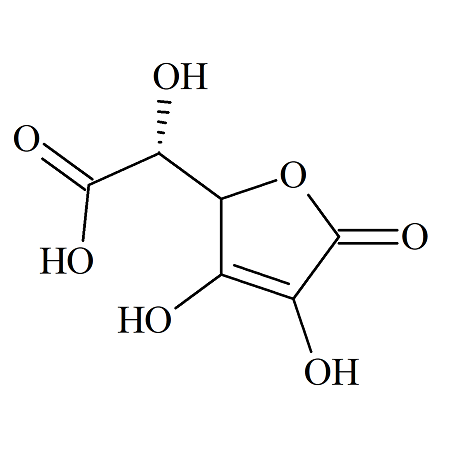
Ascorbic Acid EP Impurity G
- Product Number ASC-16-001
- Parent Drug Ascorbic Acid
- CAS Number 66757-69-5
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
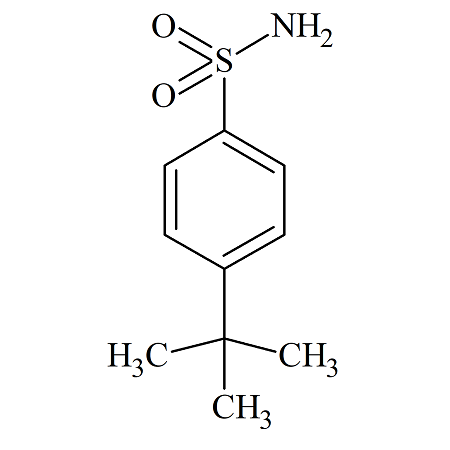
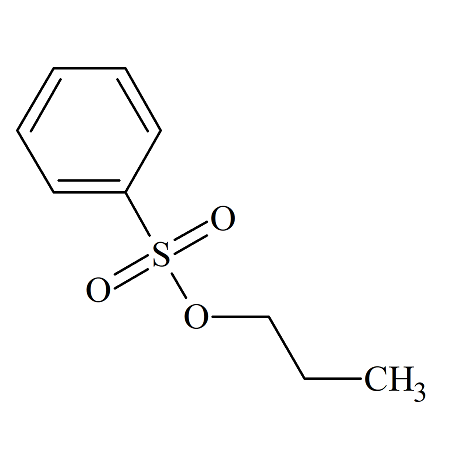
n-Propyl Benzenesulfonate
- Product Number BES-09-001
- Parent Drug Benzenesulfonates
- CAS Number 80-42-2
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options