Drug Impurities Reference Standards
Showing 1761–1770 of 1775 results
-
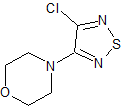
Timolol EP Impurity F
- Product Number CT-01110-844
- Parent Drug Timolol
- CAS Number 30165-96-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Eszopiclone (S-Zopiclone)
- Product Number CT-01110-570
- Parent Drug Zopiclone
- CAS Number 138729-47-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
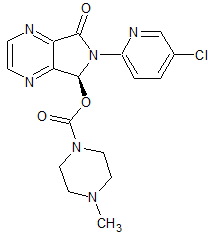
Valsartan Impurity 30
- Product Number CT-01110-135
- Parent Drug Valsartan
- CAS Number 209911-63-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
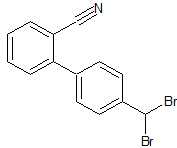
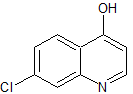
Moxifloxacin Impurity 23
- Product Number CT-01110-188
- Parent Drug Moxifloxacin
- CAS Number 154093-72-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
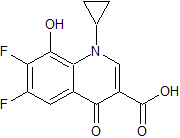
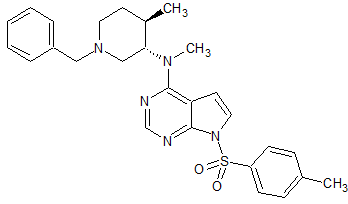
Tofacitinib Impurity 36
- Product Number CT-01110-233
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
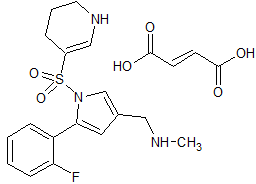
Vonoprazan Fumarate Impurity 49
- Product Number CT-01110-64
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Hydroxychloroquine Impurity 10
- Product Number CT-01110-1019
- Parent Drug Hydroxychloroquine
- CAS Number 86-99-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
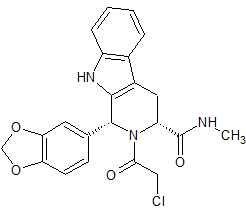
Tadalafil Impurity 24
- Product Number CT-01110-262
- Parent Drug Tadalafil
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
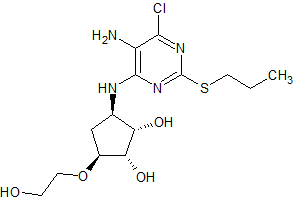
Ticagrelor Impurity 10
- Product Number CT-01110-310
- Parent Drug Ticagrelor
- CAS Number 1402150-32-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
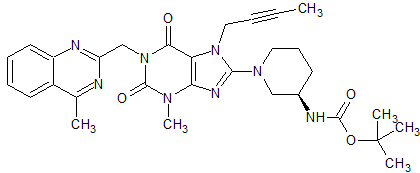
Linagliptin Impurity 46
- Product Number CT-01110-359
- Parent Drug Linagliptin
- CAS Number 668273-75-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options