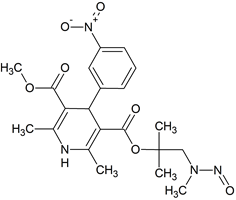Drug Impurities Reference Standards
Showing 31–40 of 1775 results
-
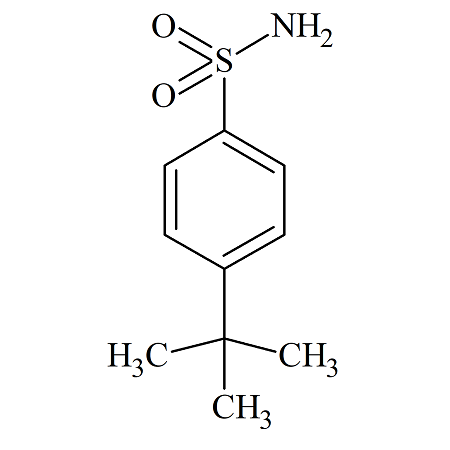
Bosentan USP RC E
- Product Number BOS-12-002
- Parent Drug Bosentan
- CAS Number 6292-59-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
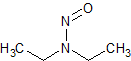
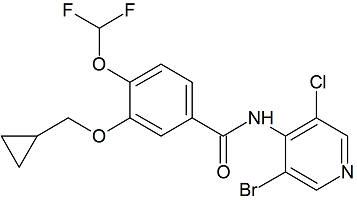
N-nitrosodiethylamine (NDEA)
- Product Number N-10521-03
- Parent Drug Nitroso Compounds
- CAS Number 55-18-5
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 to 3 week(s)See more size options -
Mirabegron N-Nitroso
- Product Number M-20224-01
- Parent Drug Mirabegron
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: week(s)See more size options -
N-Des(3,3-diphenylpropyl) Lercanidipine N-nitroso
- Product Number L-31012-01
- Parent Drug Lercanidipine
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
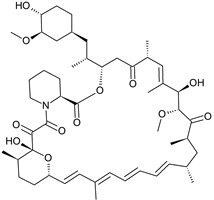
Rapamycin Tetraene Impurity
- Product Number R-41213-01
- Parent Drug Rapamycin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
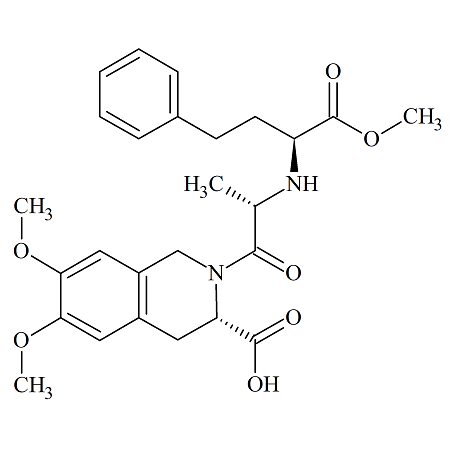
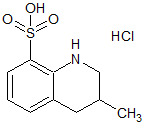
Moexipril USP RC G
- Product Number MOE-12-008
- Parent Drug Moexipril
- CAS Number 122379-46-8
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
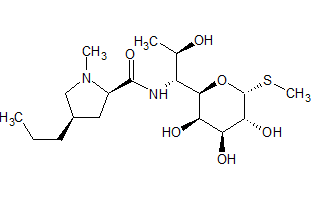
Lincomycin a-Amide Epimer
- Product Number L-90116-01
- Parent Drug Lincomycin
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Roflumilast Impurity E
- Product Number R-31123-01
- Parent Drug Roflumilast
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Argatroban Impurity 4
- Product Number CT-01110-12
- Parent Drug Argatroban
- CAS Number 153886-68-1
- Category Drug Impurities Reference Standards
Pending QCSee more size options