Drug Impurities Reference Standards
Showing 451–460 of 1775 results
-
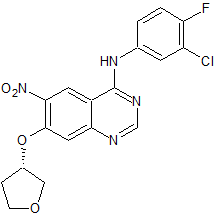
Afatinib Impurity 1
- Product Number CT-01110-03
- Parent Drug Afatinib
- CAS Number 314771-88-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
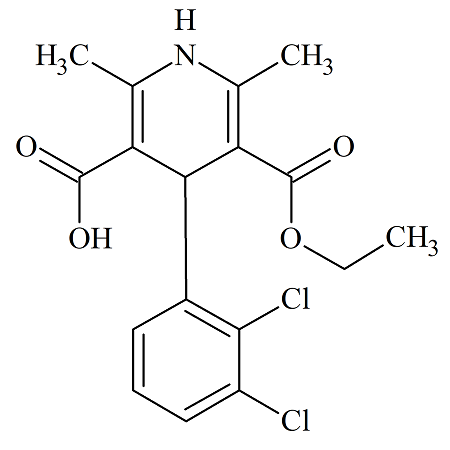
Monoethyl Felodipine
- Product Number FEL-09-007
- Parent Drug Felodipine
- CAS Number 150131-21-8
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
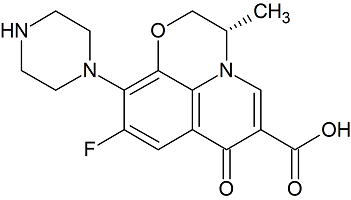
Levofloxacin Related Compound A
- Product Number L-10208-01
- Parent Drug Levofloxacin
- CAS Number 117707-40-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
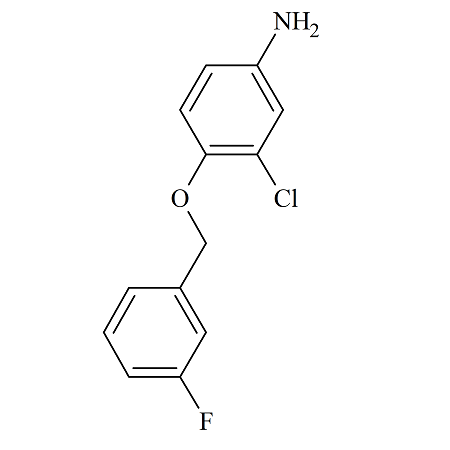
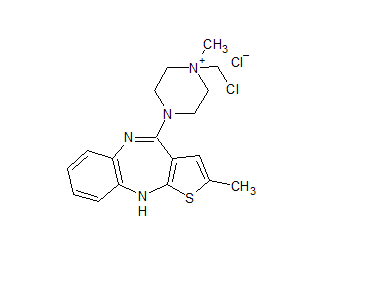
Olanzapine Chloromethyl Chloride Impurity
- Product Number OLA-20-001
- Parent Drug Olanzapine
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
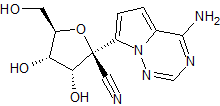
Remdesivir Desphosphate epi-Cyano Impurity
- Product Number R-00720-01
- Parent Drug Remdesivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 2 week(s)See more size options -
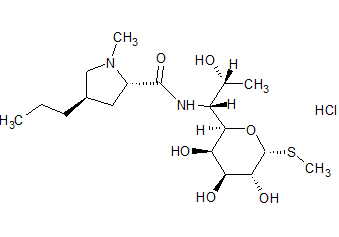
Lincomycin HCl
- Product Number ACC-171213-0001
- Parent Drug Clindamycin
- CAS Number 859-18-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Tedizolid Impurity 7
- Product Number CT-01110-124
- Parent Drug Tedizolid
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Moxifloxacin impurity 9
- Product Number CT-01110-208
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
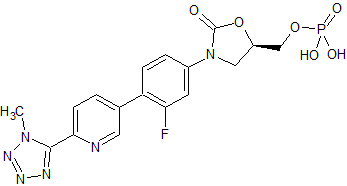
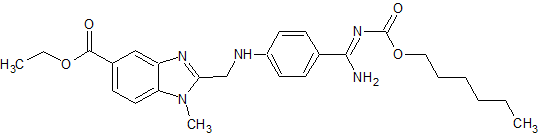
Dabigatran Impurity 4
- Product Number CT-01110-290
- Parent Drug Dabigatran
- CAS Number 1408238-36-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options