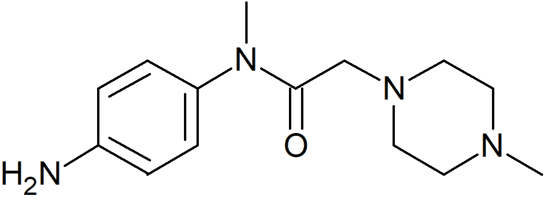Drug Impurities Reference Standards
Showing 911–920 of 1775 results
-
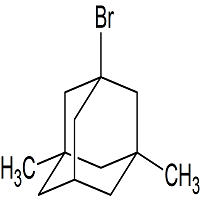
Memantine USP Related Compound D
- Product Number M-11018-01
- Parent Drug Memantine
- CAS Number 941-37-7
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
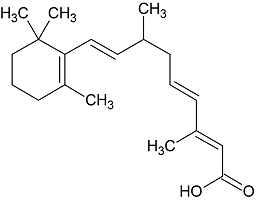
9,10-Dihydroretinoic Acid
- Product Number R-20110-01
- Parent Drug Retinoic Acid
- CAS Number 98299-56-0
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
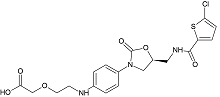
Rivaroxaban Open Ring Acid Impurity
- Product Number R-21214-02
- Parent Drug Rivaroxaban
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
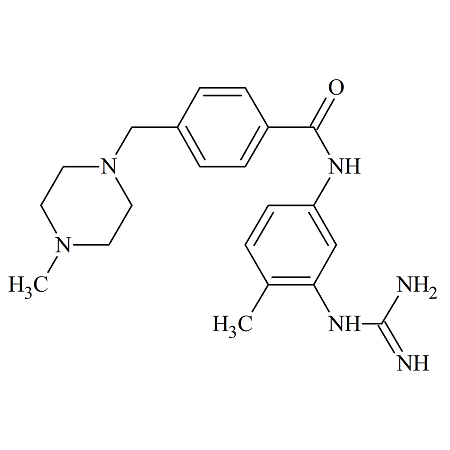
N-(4-Aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide
- Product Number N-40909-01
- Parent Drug Nintedanib
- CAS Number 262368-30-9
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
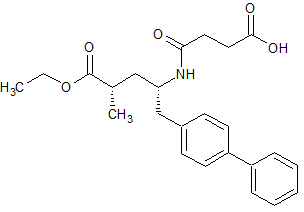
Sacubitril-(2S,4S)-Isomer
- Product Number S-90710-02
- Parent Drug Sacubitril
- CAS Number 149709-63-7
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
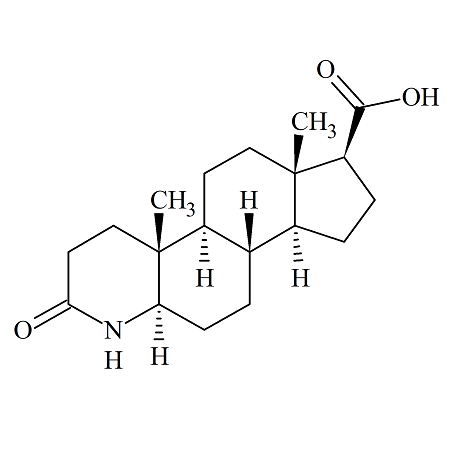
Dutasteride Acid Dihydro
- Product Number ACB-170323-0006
- Parent Drug Dutasteride
- CAS Number 103335-55-3
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
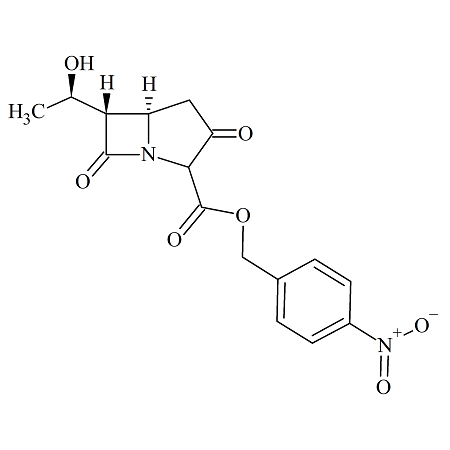
Imipenem intermediate: p-Nitrobenzyl Ester
- Product Number IMP-16-001
- Parent Drug Imipenem
- CAS Number 74288-40-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options