Drug Impurities Reference Standards
Showing 91–100 of 1775 results
-
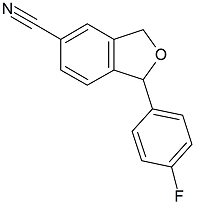
1-(4-Fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile
- Product Number C-30106-01
- Parent Drug Citalopram
- CAS Number 64169-67-1
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
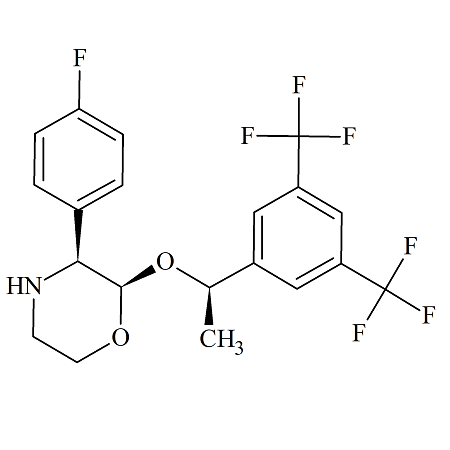
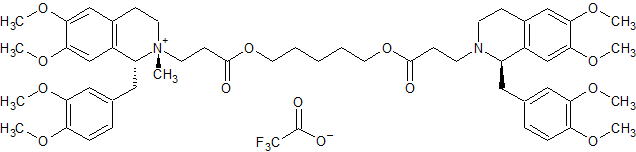
Aprepitant Stage-II (Impurity-A)
- Product Number ACA-160919-0002
- Parent Drug Aprepitant
- CAS Number 171482-05-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
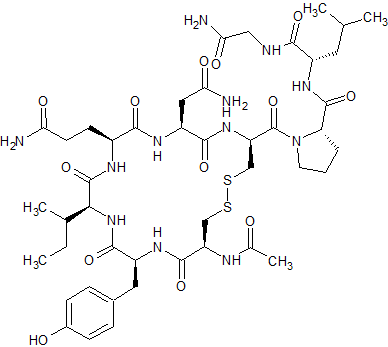
Ac-Cys1-Oxytocin
- Product Number CT-01110-1115
- Parent Drug Oxytocin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
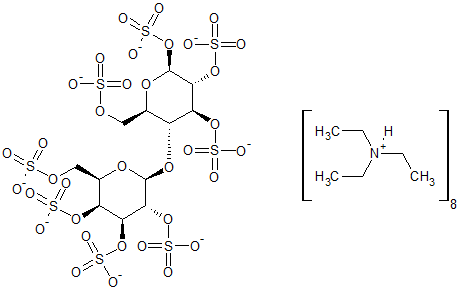
Lactose Octasulfate Potassium Salt
- Product Number S-90429-04
- Parent Drug Sucralfate
- CAS Number N/A
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options -
Atracurium EP Impurity Q
- Product Number CT-01110-1039
- Parent Drug Atracurium
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
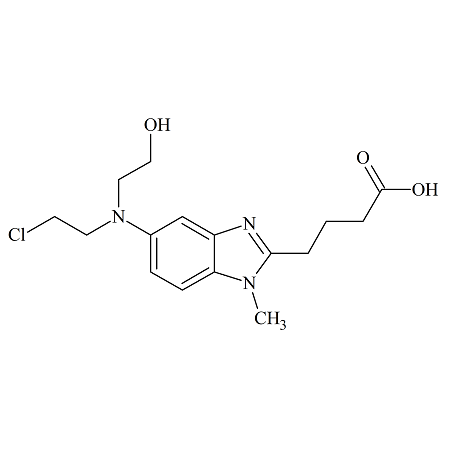
Olmesartan Medoxomil Dehydro
- Product Number OLM-10-002
- Parent Drug Olmesartan
- CAS Number 879562-26-2
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 4 to 6 week(s)See more size options -
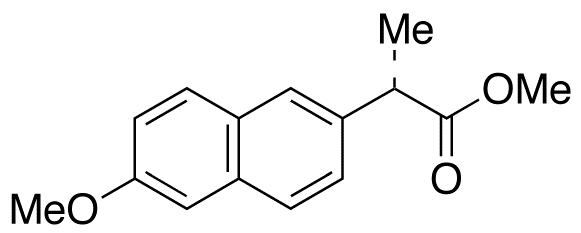
Naproxen Methyl Ester
- Product Number NAP-16-002
- Parent Drug Naproxen
- CAS Number 26159-35-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
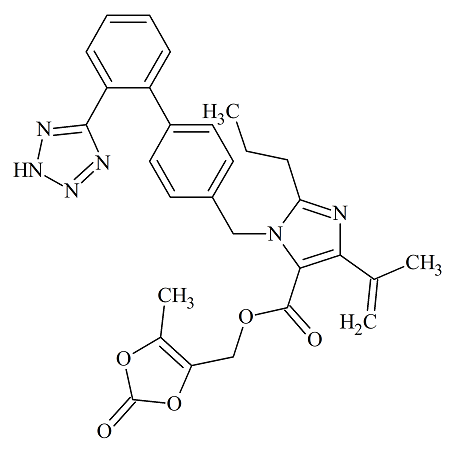
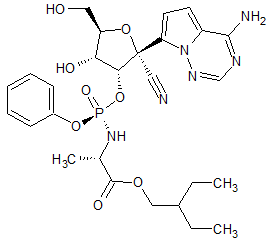
Remdesivir 2′-Phosphate Isomer
- Product Number R-00720-23
- Parent Drug Remdesivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Made to Order - Lead Time: 6 week(s)See more size options -
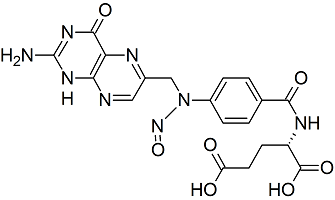
Folic Acid N-Nitroso
- Product Number F-30318-01
- Parent Drug Folic Acid
- Category Drug Impurities Reference Standards
In Stock - Ready to ShipSee more size options