Drug Impurities Reference Standards
Showing 111–120 of 1775 results
-
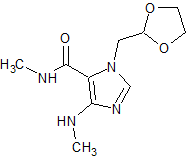
Doxofylline Impurity 1
- Product Number CT-01110-862
- Parent Drug Doxofylline
- CAS Number 1429636-74-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
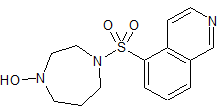
Fasudil Impurity 9
- Product Number CT-01110-678
- Parent Drug Fasudil
- CAS Number 1350827-92-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
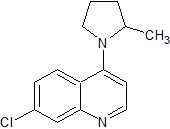
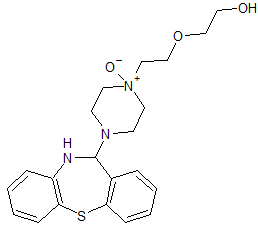
Hydroxychloroquine EP Impurity F
- Product Number CT-01110-1023
- Parent Drug Hydroxychloroquine
- CAS Number 6281-58-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
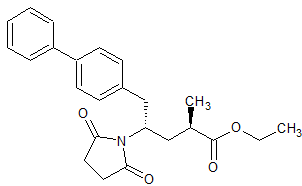
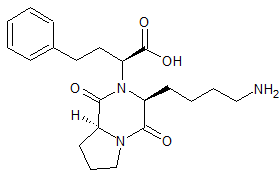
LCZ-696 Impurity 4
- Product Number CT-01110-946
- Parent Drug LCZ-696
- CAS Number 1038924-97-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Lisinopril EP Impurity C
- Product Number CT-01110-932
- Parent Drug Lisinopril
- CAS Number 328385-86-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
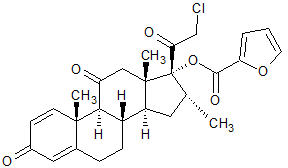
Mometasone Furoate EP Impurity C
- Product Number CT-01110-955
- Parent Drug Mometasone
- CAS Number 1305334-31-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
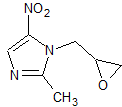
Ornidazole Impurity 9
- Product Number CT-01110-643
- Parent Drug Ornidazole
- CAS Number 16773-51-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Pioglitazone EP Impurity C
- Product Number CT-01110-714
- Parent Drug Pioglitazone
- CAS Number 952188-00-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
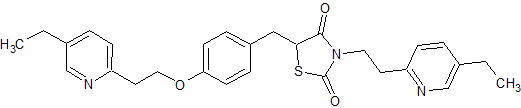
Quetiapine EP Impurity H
- Product Number CT-01110-591
- Parent Drug Quetiapine
- CAS Number 1076199-40-0
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
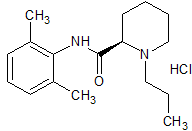
Ropivacaine EP Impurity G
- Product Number CT-01110-974
- Parent Drug Ropivacaine
- CAS Number 112773-90-7
- Category Drug Impurities Reference Standards
Pending QCSee more size options