Drug Impurities Reference Standards
Showing 991–1000 of 1775 results
-
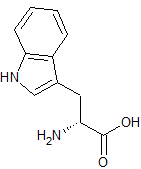
Prilocaine EP Impurity D
- Product Number CT-01110-1084
- Parent Drug Prilocaine
- CAS Number 878791-35-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
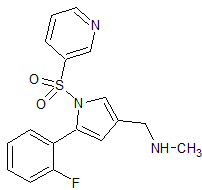
Rifaximin EP Impurity F
- Product Number CT-01110-859
- Parent Drug Rifaximin
- CAS Number 14487-05-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
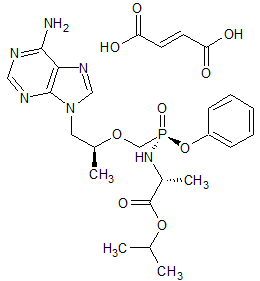
Tenofovir Alafenamide Impurity 6
- Product Number CT-01110-993
- Parent Drug Tenofovir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
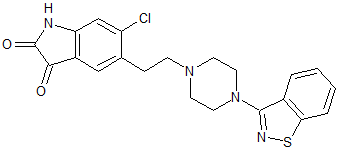
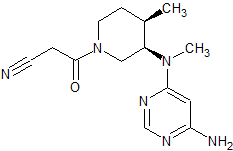
Ziprasidone EP Impurity B
- Product Number CT-01110-575
- Parent Drug Ziprasidone
- CAS Number 1159977-56-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Valsartan Impurity 25
- Product Number CT-01110-133
- Parent Drug Valsartan
- CAS Number 133051-88-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
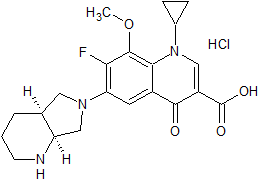
Moxifloxacin Impurity 15
- Product Number CT-01110-185
- Parent Drug Moxifloxacin
- CAS Number 2205053-60-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tofacitinib Impurity 33
- Product Number CT-01110-231
- Parent Drug Tofacitinib
- CAS Number 1640971-60-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Vonoprazan Fumarate impurity 47
- Product Number CT-01110-62
- Parent Drug Vonoprazan
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
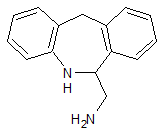
Epinastine Impurity 10
- Product Number CT-01110-1028
- Parent Drug Epinastine
- CAS Number 41218-84-2
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tadalafil Impurity 18
- Product Number CT-01110-259
- Parent Drug Tadalafil
- CAS Number 153-94-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options