Drug Impurities Reference Standards
Showing 961–970 of 1775 results
-
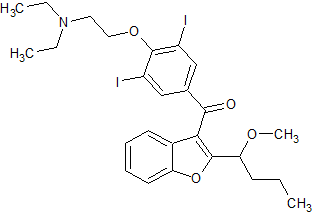
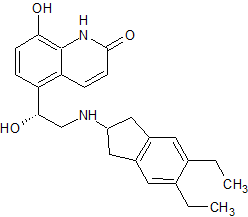
Amiodarone EP Impurity G(HCl)
- Product Number CT-01110-779
- Parent Drug Amiodarone
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
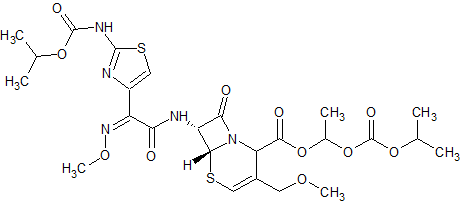
Cefpodoxime Proxetil Impurity 8
- Product Number CT-01110-881
- Parent Drug Cefpodoxime
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Elagolix Impurity 4
- Product Number CT-01110-1118
- Parent Drug Elagolix
- CAS Number 2316733-82-9
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
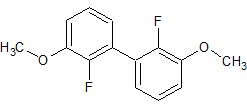
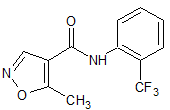
Flumazenil EP Impurity E
- Product Number CT-01110-788
- Parent Drug Flumazenil
- CAS Number 78756-03-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
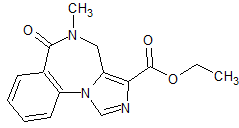
Indacaterol Impurity 7
- Product Number CT-01110-853
- Parent Drug Indacaterol
- CAS Number 1403389-05-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Leflunomide EP Impurity F
- Product Number CT-01110-914
- Parent Drug Leflunomide
- CAS Number 1403564-06-6
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
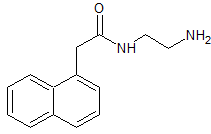
Loratadine 2-Hydroxymethyl Impurity (USP)
- Product Number CT-01110-782
- Parent Drug Loratadine
- CAS Number 609806-39-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
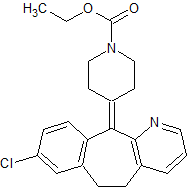
Naphazoline EP Impurity A
- Product Number CT-01110-801
- Parent Drug Naphazoline
- CAS Number 36321-43-4
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
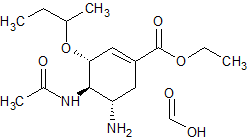
Oseltamivir EP Impurity F formate
- Product Number CT-01110-964
- Parent Drug Oseltamivir
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
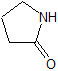
Piracetam EP Impurity A
- Product Number CT-01110-563
- Parent Drug Piracetam
- CAS Number 616-45-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options