Drug Impurities Reference Standards
Showing 1041–1050 of 1775 results
-
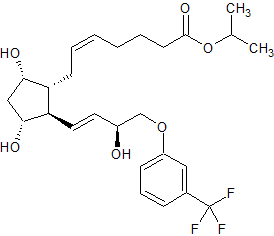
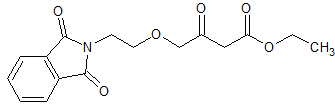
Travoprost Impurity 4
- Product Number CT-01110-1081
- Parent Drug Travoprost
- CAS Number 1420791-14-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
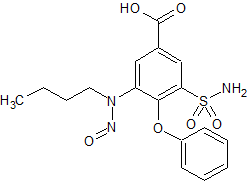
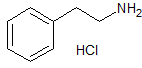
Bumetanide N-Nitroso Impurity
- Product Number B-10312-02
- Parent Drug Bumetanide
- CAS Number 2490432-02-3
- Category Drug Impurities Reference Standards
Temporarily Out of StockSee more size options -
Aripiprazole EP Impurity G
- Product Number CT-01110-15
- Parent Drug Aripiprazole
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Moxifloxacin Impurity 48
- Product Number CT-01110-199
- Parent Drug Moxifloxacin
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
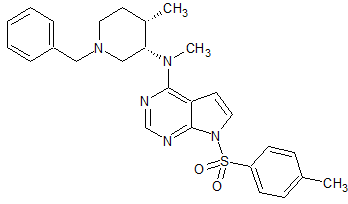
Tofacitinib Impurity 64
- Product Number CT-01110-244
- Parent Drug Tofacitinib
- CAS Number N/A
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Mirabegron Impurity 20
- Product Number CT-01110-80
- Parent Drug Mirabegron
- CAS Number 156-28-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Nicorandil EP Impurity C HCl
- Product Number CT-01110-1061
- Parent Drug Nicorandil
- CAS Number 72676-16-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Tadalafil Impurity 45
- Product Number CT-01110-272
- Parent Drug Tadalafil
- CAS Number 36060-94-3
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
Amlodipine Impurity27
- Product Number CT-01110-326
- Parent Drug Amlodipine
- CAS Number 88150-75-8
- Category Drug Impurities Reference Standards
Pending QCSee more size options -
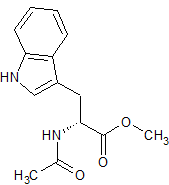
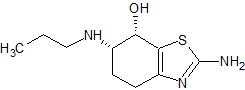
rac-cis-7-Hydroxy-Pramipexole ( and enantiomer )
- Product Number CT-01110-376
- Parent Drug Pramipexole
- CAS Number 1798014-87-5
- Category Drug Impurities Reference Standards
Pending QCSee more size options